Giant mucinous cystadenoma: case report with review of literature
Case summery
Radiological imaging may sometimes not be specific about cystic lesions in and around pancreas and a cystic tumor may be wrongly diagnosed as hydatid cyst or a pseudocyst. Analysis of cystic fluid aspirate has improved diagnosis of pancreatic cystic lesions significantly. Biopsy and histology of cyst wall is diagnostic and should be considered in cases with inconclusive preoperative diagnosis and questionable intra operative findings. We present a patient with a huge mucinous cystadenoma of the pancreas initially mistaken for a pancreatic hydatid cyst though she was sero negative for hydatid.
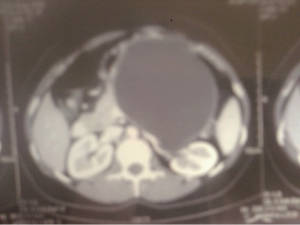
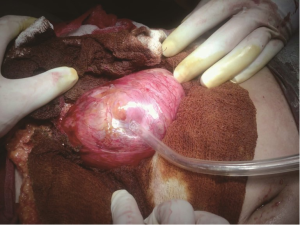
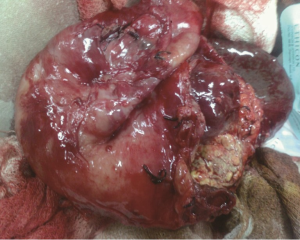
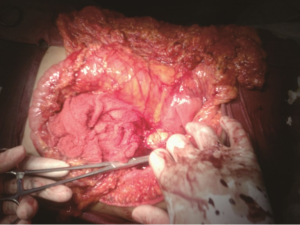
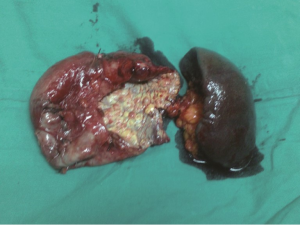
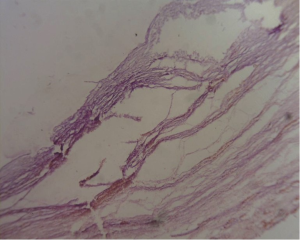
A 30-year-old lady presented with 4 months history of back pain radiating towards the epigastrium. She also noticed a gradually increasing abdominal distension. Her bowel and bladder habits were normal. She had no previous history of abdominal pains or jaundice. She had no history of hospitalization for severe pain or acute pancreatitis. She had a firm, non-tender central abdominal mass occupying the upper 1/3 of the abdomen. Abdominal ultrasonography and computed tomography (CT) scan (Figure 1) was suggestive of cystic lesion in relation to tail of pancreas. A tentative diagnosis of pancreatic hydatid cyst was made as hydatid cyst is very common in Kashmir valley. Her serum amylase, liver function test, kidney function test, blood sugar and other relevant hematological parameters were normal. On laparatomy there was a huge retroperitoneal cyst displacing the stomach upwards and the small bowel into the pelvis. Once abdomen was walled off with betadine soaked packs to avoid any spillage (Figure 2); the cyst was observed to be thick walled to be incised and contained a thick viscid clear fluid. An on table decision to excise in toto was made considering a diagnosis of cystic tumor of pancreas. A hemipancreatectomy beyond healthy pancreatic margin with splenectomy was performed (Figures 3,4). Tumor arose from the tail of pancreas and weighed about 2.5 (Figure 5). Aspirated fluid was sent for investigation was mucin positive and CEA and CA 19-9 were normal. Histologically she was diagnosed to have potentially benign mucinous cystadenoma (Figure 6).
She had post-operative fever that was managed with parenteral antibiotics and was discharged twelve days after surgery.
She has been followed up for more than 5 years now and has remained well.
Discussion
Cystic neoplasm’s of the pancreas are rare and comprise 10% to 15% of pancreatic cystic masses and only 1% of pancreatic cancers (1,2). They are slow growing indolent tumors with low-grade malignant potentials commonly seen in middle aged women. Commonly asymptomatic, they sometimes reach large sizes prior to diagnosis. Routine use of abdominal ultrasound, CT and MR has led to an increase in detection of pancreatic cystic lesions and reduced the average size at diagnosis (3). Pancreatic cystic neoplasm’s consist of mucinous cystic neoplasm’s which commonly arise from the body and tail of the pancreas, serous cystic neoplasm’s that are almost always benign and is anywhere along pancreas, and intraductal papillary mucinous tumors (IPMT) and unusual cystic neoplasms including cystic islet cell tumors. Mucinous cystic neoplasms include mucinous cystadenomas (65%), proliferative cystic mucinous neoplasms (30%) and mucinous cystadenocarcinomas (5%) (4). Misdiagnosis and maltreatment of pancreatic neoplasms as pseudocysts is not uncommon (5). Lack of a history of trauma, chronic pancreatitis or a recent history suggestive of acute pancreatitis should raise a possibility of a cystic neoplasm of the pancreas. Up to 40-75% of patients with cystic pancreatic tumors are asymptomatic (6), and majorities are detected incidentally on imaging modalities. Though a tertiary health care center like ours should have a high index of suspicion for cystic tumor but as in our place hydatid cyst is endemic, it overweighed our suspicion. A final diagnosis was established on table. In the era of advanced imaging with CT and MRI; MRI should be to accurately differentiate a cystic neoplasm from other cystic pathology. Percutaneous or endoscopic ultrasonography with guided aspiration and analysis of cystic fluid for amylase activity, carcinoembryonic antigen level, viscosity, presence of mucin, cytology, tumor markers and DNA analysis improves diagnosis significantly (7). Carcino-embryonic antigen level estimation can be very sensitive in diagnosis of mucinous cystic tumors (8). IPMNs are found most often in male patients in their 60 or 70 s, and are more often than not found in the pancreatic head/neck region. IPMNs appear “grape-like” on imaging, including on endoscopic ultrasound (EUS) and appear as cysts side by side one another rather than the “Cyst within a cyst” characteristically seen in MCNs. IPMN lesions also communicate with the pancreatic duct, a feature not seen in MCNs. MCNs in comparison, are often seen in females in the 40- to 50-year age range and are located most often in the pancreatic body and tail regions.
Intraoperative, cystic neoplasm’s are thick walled and usually contain clear fluid, while pseudo cysts fluid is usually grey, opalescent and contain blood or necrotic debris, whereas hydatid cyst will have a membrane with daughter cysts. Biopsy of cyst wall with frozen section and histology is diagnostic. The currently accepted guidelines are that for serrous cystic tumor is an organ-preserving resection, although there are proponents of a conservative line of management (9,10). A fair consensus is that lesions that are asymptomatic and/or <4 cm in size can be followed up at yearly intervals, while surgery should be offered to patients with symptomatic lesions and tumours >4 cm, because they have been seen to grow at a rate of almost 1.98 cm/year (11).
In the case of mucinous tumors, a more radical resection is advised. This is based on the understanding of its malignant potential (9,10). As these tumors usually occur in the fourth to fifth decade, the likelihood of a malignant transformation is also high. In the presence of predictors of malignancy, such as large tumor size, mural nodules and egg-shell calcification, spleen preserving techniques should be avoided to obtain a correct oncological lymph node dissection (12). Recent improvement in morbidity and mortality of pancreatic surgery has encouraged complete excision in patients with cystic tumors of pancreas.
Complications of tumor resection include pancreatic fistula, portal vein thrombosis, abscesses, and hemorrhage and so should be considered in hands of experts. An improved technique of evaluation is now leading to a specific diagnosis in cystic tumors of pancreas. One should follow proposed guidelines of managing these tumors and resect all deserving cystic lesions (6).
Conclusions
Cystic neoplasm’s are rare but should be considered in cystic lesions around the pancreas. An absence of history of trauma or history suggestive of inflammation of the pancreas may suggest a cystic tumor necessitating a detailed preoperative evaluation. Prognosis of cystic mucinous tumors of pancreas is favorable and should be operated timely.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hodgkinson DJ. ReMine WH, Weiland LH. Pancreatic cystadenoma. A clinicopathologic study of 45 cases. Arch Surg 1978;113:512-9. [PubMed]
- Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg 1999;178:269-74. [PubMed]
- Friedman AC, Lichtenstein JE, Dachman AH. Cystic neoplasms of the pancreas. Radiological-pathological correlation. Radiology 1983;149:45-50. [PubMed]
- Warshaw AL, Compton CC, Lewandrowski K, et al. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg 1990;212:432-43; discussion 444-5. [PubMed]
- Warshaw AL, Rutledge PL. Cystic tumors mistaken for pancreatic pseudocysts. Ann Surg 1987;205:393-8. [PubMed]
- Hutchins GF, Draganov PV. Cystic neoplasms of the pancreas: a diagnostic challenge. World J Gastroenterol 2009;15:48-54. [PubMed]
- Sarr MG, Kendrick ML, Nagorney DM, et al. Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. Surg Clin North Am 2001;81:497-509. [PubMed]
- Cizginer S, Turner BG, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011;40:1024-8. [PubMed]
- Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg 2003;7:417-28. [PubMed]
- Lim SJ, Alasadi R, Wayne JD, et al. Preoperative evaluation of pancreatic cystic lesions: cost-benefit analysis and proposed management algorithm. Surgery 2005;138:672-9; discussion 679-80. [PubMed]
- Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg 2005;242:413-9; discussion 419-21. [PubMed]
- Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg 1998;227:896-903. [PubMed]