Exploring breast with therapeutic ductoscopy
Introduction
Nipple discharge is the third most common complaint among women, often prompting referral to a surgeon, but only rarely a sign of underlying malignancy. Pathologic nipple discharge (PND) is defined as spontaneous or easily expressible single duct nipple discharge that contributes to 5% of referrals to breast surgeons (1,2). Patients with PND represent a diagnostic and therapeutic challenge for the surgical clinician. The most frequent causes of PND in these cases are intraductal papilloma (IP) in 36% to 66% (3-8), ductal carcinoma in situ (DCIS) in 3% to 20% (3-7,9) and other benign causes in up to 23% (4,5). The evaluation of women with PND usually involves radiological (mammography, ultrasound, ducto (galacto) graphy and cytological (nipple smear, ductal lavage) examinations (4,9,10). Clinical uncertainty arises in the patient with PND including no abnormality on exam or imaging. Despite the low likelihood of malignancy, these women require surgical excision of the duct(s) to make the diagnosis, either via a central or a single duct excision. This recommendation is based on the inadequate sensitivity and specificity of imaging or of additional tests, such as cytological examination of the discharge or ductography (11). However, much of these data originates from a time when breast imaging was perhaps less sensitive or without an emerging intraductal technique known as ductoscopy. A ductoscope is an instrument which allows visualization of abnormalities and polypoid lesions such as papilloma within the ductal system with access via the nipple orifice to aid in evaluation of PND. Moreover, it is currently being used to improve localization of lesions in patients with PND. Ductoscopically guided excision is an improvement over standard surgical approaches with terminal duct excision which removes a large volume of tissue with potential cosmetic deformity. Ductoscopy also allows retrieval of intraductal cells or tissues for diagnostic purposes using biopsy forceps/basket or brush cytology (6,12,13). Recently, therapeutic endoscopic lesion extractions were also performed by the development of those biopsy tools. Moreover, ductoscopy does not require general anesthesia while diagnostic exploration and ductoscopic papilloma extraction. However, during ductoscopy assisted microductectomy, having general anesthesia is better option for patient comfort.
Current ductoscopy systems with endoscopic biopsy option
The lack of robust diagnostic options and the desire to directly visualize and biopsy the intraductal lesions producing PND led to the development of ductoscopy. After initial blind intraductal biopsy experiences, rigid endoscopes were developed. With the efforts of researchers in the early 1990s, it became possible to directly visualize inside the breast ducts. In 1988, Teboul used a 1.7 mm rigid ductoscope and ultrasonography to observe the ductal cavity (14). Ductoscopy helped to limit the extent of surgery and provided a tissue-conserving approach.
In contrast to initial rigid ductoscopes with a diameter of more than 1.5 mm, today’s technology has given us the opportunity to use fiber-optic ductoscopes with smaller diameters.The optical instruments gained higher resolution, and working channels were developed that allow the surgeons perform direct biopsies (15).
Several ductoscopes have been approved for use by the FDA. According to the FDA, a ductoscope is a device intended for use in viewing an interior cavity of the human body through either a natural opening or an incision. Examples of these devices include the ViaDuct™ Miniscope (Acueity Inc., Palo Alto, CA, USA) and the Mastascope™ (Lifeline Biotechnologies, Pompano Beach, FL, USA). The Viaduct mammary ductoscope has high quality fiber optics with an external diameter of 0.9 mm, which has a 0.2-mm secondary channel attachment for insufflation and tissue sampling for diagnosis. A gas sterilizable fiber-optic core is placed within the sheath and connected to a video monitor with 60× magnification. It has been observed that distal ducts can be successfully scoped if ductal discharge is observed at the time of endoscopy (45% versus 0%) (12). Sterile saline is used for ductal distention, thus avoiding the air-fluid interface and optimizing the distention while keeping the ductal system intact (12). Nevertheless, Viaduct mammary ductoscope does not include a working channel to insert a wire with a basket to do extraction of the polypoid lesions. However, a European scope known as Laduscope has a working channel to insert instruments into the breast ducts under direct imaging (15).
Several techniques are currently used for transductal endoscopic tissue sampling, including endoscopic vacuum-assisted biopsy (16). Using this method, ductoscopy was performed with a rigid 0.7 mm gradient index microendoscope (Volpi AG, Schlieren, Switzerland), and ductoscopic biopsy was successfully performed in 34 of 36 patients with nipple discharge and intraductal lesions. The size of the biopsy specimen was approximately 1 mm, and the quality of the samples was adequate for histopathologic analysis and immunochemistry. Sufficient diagnostic material was obtained by intraductal biopsy in approximately 90% of the patients, and histopathology showed benign papilloma in most patients (n=26). Thus, vacuum-assisted biopsy techniques may be used therapeutically because small lesions can be removed completely by repeated biopsies.
Intraductal breast biopsy (IDBB) examination under mammoscopic observation has been used for pathologic diagnosis of intraductal lesions accompanied by nipple discharge (17). The outer diameters of the mammoscopes were 0.55, 0.7, and 0.72 mm (Fiber Tech Company, Tokyo, Japan), and all were semirigid endoscopes. This procedure was performed in 107 patients with a total of 193 IPs and in 27 patients with 30 ductal carcinomas. This procedure showed therapeutic efficacy in 54 of 70 patients with IP (77.6%). Thus, IDBB examination represents a new tool in the diagnosis and treatment of secreting benign intraductal lesions and should be evaluated further in multicenter studies. Additionally, IDBB will allow the future development of studies to treat pre-invasive atypia and DCIS with administration of intraductal medications and/or energy sources. IDBB would allow for pre and post treatment biopsies to assess treatment efficacy.
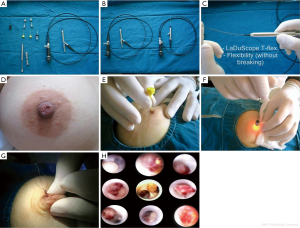
To date, there has been only one published article describing the complete endoscopic retrieval of a solitary papilloma (Figure 1A-H) (15). Of the 102 patients from this study, 26 (25.4%) had solitary papillomas, 5 (4.9%) had multiple papillomas, 11 (10.7%) had intraductal debris and 1 had a ductal epithelial surface abnormality with positive cytology. Only one patient had subsequent mastectomy after a positive surgical margin with ductoscopy-aided microductectomy. The mean ± SD examination time was 23.5±14.4 min (range, 7-110 min) in lesion-free patients and 36.6±26.1 min (range, 13-110 min) in the patients who underwent endoscopic papillomectomy. Most studies have described the partial removal of polypoid lesions, followed by repetition of the procedure. Moreover, little is known about the volume of remnant tissue after endoscopic biopsy (16,17). Ductoscopic papillomectomy (DP), which is recently described by Bender et al., has shown to have a potential on diagnosing papilloma which causes PNDs and also a therapeutic efficacy of 95.4% (15). In this study, most of the visualized polypoid lesions histopathologically confirmed as papilloma were completely removed endoscopically. Neither recurrence of discharge nor development of malignancy was reported. To date, transductal DP is the only scarless intervention of the breast through the natural nipple orifice as well as the only surgical intervention that preserves breast milking function. Technical improvements in interventional ductoscopies should increase the therapeutic efficacy and diagnostic potential of those on benign and malignant lesions via intraductal access.
Technical difficulties and uncommon complications
Potential complications of ductoscopy are uncommon and include pain, inflammation, and infection. Occasionally, ductoscopy fails as a result of luminal occlusion from scarring and sclerosis. Access to peripheral lesions may be limited by the scope length (6-8 cm). PND, which is coming spontaneously from a single duct facilitates surgeon to identify the correct orifice of the interesting duct. The duct has generally wide opening than the others which makes possible for visual identification. However, this could not prevent the surgeon from having false rupture of the duct. Training period as 5 to 10 attempt of the ductoscopic exploration would be appropriate to have orientation of the procedure. Perforation of the duct by the scope creates a false passage into the breast parenchyma and usually can be recognized by transition of the visual image of ducts from a white shiny smooth surface to a gray ragged surface (fibrous parenchyma) or to a yellow cavernous honeycomb (adipose tissue). Narrow ductal orifices or acute angulation of ductal branches may predispose to duct perforation. No secondary or long-lasting effects of breast duct perforation have been reported, although this may be a transient cause of postprocedure discomfort (18). Furthermore, polypoid lesions near the nipple would make easier the DP rather than those far from the nipple. Far lesions would be challenging for extraction endoscopically due to the difficulty during the advance of the shaft in the narrow ducts.
Ductoscopic management of PND and microductectomy
Despite the low likelihood of malignancy, it is recommended that all women with PND undergo duct excision based on the inadequate sensitivity of diagnostic modalities. However, these data originates prior to recent improvements in breast imaging. Recently, Sabel et al. (11) University of Michigan Comprehensive Cancer Center performed a retrospective review of patients evaluated in the setting of modern diagnostic breast imaging. Of 175 women referred to their breast clinic with a primary complaint of nipple discharge, 142 (81%) had suspicious (pathologic) discharge. Of the 23 patients who opted for observation over duct excision, with a mean follow-up of 3.3 years, none have been diagnosed with cancer. Among patients who proceeded with surgery, cancer was diagnosed in seven patients (5%). Six of the seven patients had either an abnormal mammogram or ultrasound. Among 46 patients with suspicious nipple discharge, a normal physical exam and normal diagnostic mammogram/ultrasound, only one malignancy (2%) was identified in a 79-year-old patient with a personal history of breast cancer. Sabel et al. concluded that in selected patients with suspicious nipple discharge, but normal physical exam and diagnostic imaging, short-term observation with repeat evaluation seems reasonable for patients who do not desire duct excision. Recently, Balci et al. (19) reported success on DP without recurrence of discharge or developed malignancy at 5 years follow-up. Thus, papilloma extraction could be oncologically safe while performed ductoscopically which provides rapidly disappearing of PND.
The method of evaluation of PND varies greatly among physicians and within the literature. In general, most women presenting with PND will be evaluated initially with mammogram and/or ultrasound, depending on her age and physician preference. With any mammographic or sonographic abnormalities, there is a significantly increased risk of cancer (3). Most women with PND, however, will have normal imaging. Additional imaging that may be considered includes ductography or magnetic resonance imaging (MRI). However, often physicians proceed to major duct excision or lacrimal-probe-guided duct excision when routine imaging is unremarkable. Whereas lacrimal-probe guidance may help the surgeon perform a more directed approach, it does not permit visualization of the intraductal system. Patients where the intraductal pathology was up to 8-10 cm deep to the nipple might not be resected if a “blind” terminal duct excision was performed. With this in mind, ductoscopy evolved as both a diagnostic and therapeutic tool in the setting of PND. Ductoscopically guided excision is an improvement over standard surgical approaches such as terminal duct excision, which removes a large volume of tissue with potential cosmetic deformity and for young women may make breast feeding not possible. When disposable scopes are used, the sheath of the MD may be sutured in place and if not dislodged, will enable the pathologist to identify accurately the lesion of interest to the endoscopist. It also is possible to inject methylene blue dye through the endoscope to mark the target duct for surgical excision.
Diagnostic ductoscopy for premalignant/malignant lesions
It is well established that ductoscopy is enormous for the diagnosis of solitary and multiple papillomas. However, not much consensus has been made on the diagnosis of premalignant lesions such as atypical ductal hyperplasia or DCIS. Despite ductoscopic characteristics of those lesions, it is not always possible to make a final diagnosis based on visual appearances alone.
A number of studies have evaluated endoscopic clinical features compared with histologic outcomes that give an indication of diagnostic accuracy based on visual appearances alone. Moncrief et al. (6) conducted a study comparing ductoscopy-guided duct excision with conventional terminal duct excision for 117 women with SND. In the ductoscopy-guided excision group, if ductoscopy identified a lesion, the extent of the disease was marked out on the skin by transillumination at the most proximal and most distal lesions. The outer cannula of the scope was left in place, and resection of the diseased duct was performed. For the 59 women who underwent ductoscopy-guided excision, 49 lesions were described as papillomas, but only 36 (73%) were confirmed to be papillomas at final histology. Of the lesions incorrectly described as papillomas, five were DCIS, three were atypical ductal hyperplasia (ADH), three were hyperplasias of the typical type, and two had a nonproliferative pathology. Conversely, of the 37 papillomas diagnosed pathologically, 36 (97.3%) were correctly identified by ductoscopy. The single papilloma missed was in a duct with a stricture too tight to allow the scope to pass. One patient who had DCIS confirmed by final pathology had red patches seen at ductoscopy.
Louie et al. (20) studied 188 women who underwent ductoscopy-guided excisional biospy for SND. An intraareolar incision was made, and a standard wedge biopsy was performed around the tip of the scope positioned at the most distal intraluminal defect. The final pathology was DCIS for 12 patients and invasive breast cancer for 2 patients. Ductoscopy identified intraluminal growths in 6 (43%) of these 14 patients. Other pathologies were strictures, obstruction, and wall irregularities. The remaining two patients had unremarkable ductoscopies.
It may be possible to improve the ability of ductoscopy to indicate a diagnosis based on visual appearance alone using autofluorescence technology (21). When tissues are illuminated with short-wavelength light (380-430 nm), the absorbed energy is emitted as light at a longer wavelength (475-800 nm) and observed as fluorescent light of a different color. The end result is an enhanced image that allows the operator to distinguish potentially between benign and malignant lesions or to identify early premalignant lesions such as DCIS. However, this method is of limited and also not well established by other studies.
The diagnostic accuracy of ductoscopy plus in vivo iodine staining for intraductal proliferative lesions of the breast was studied by Feng et al. (22). Following PAS staining, benign mammary hyperplasia lesions were positively stained, while negligible PAS positivity was observed in the DCIS lesions (P<0.05). Following in vitro iodine staining, benign mammary hyperplasia specimens appeared dark brown, whereas DCIS samples appeared significantly lighter or unstained. Compared with the pathological examination results, ductoscopy with iodine staining showed an agreement rate in the diagnosis of ductal intraepithelial neoplasia (DIN), sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and Youden index of 97.82%, 98.83%, 83.33%, 5.93, 0.014, and 0.8216, respectively; the corresponding values for ductoscopy without iodine staining were 88.24%, 89.16%, 50.00%, 1.78, 0.217, and 0.3916, respectively. Ductoscopy with iodine staining was found to be superior to conventional ductoscopy for the diagnosis of DIN and valuable for breast cancer prevention.
Technical studies on miniaturizing or upgrading endoscopic instruments such as biopsy tools, higher resolution cameras, and laser/radiofrequency ablators would allow improving the diagnostic and therapeutic efficacy of intraductal approach on breast lesions. More technical advances are needed to proceed with ductoscopy on the management of PND. Discharges with malignant final pathology might carefully be explored by surgeons to aid prediction of premalignant or malignant lesions during ductoscopic exploration. Intraductal therapy with oncological agents is currently of limited (23), however have potential for future intraductal studies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fisher CS, Margenthaler JA. A look into the ductoscope: its role in pathologic nipple discharge. Ann Surg Oncol 2011;18:3187-91. [PubMed]
- Dixon JM, Mansel RE. ABC of breast diseases. Symptoms assessment and guidelines for referral. BMJ 1994;309:722-6. [PubMed]
- Gülay H, Bora S, Kìlìçturgay S, et al. Management of nipple discharge. J Am Coll Surg 1994;178:471-4. [PubMed]
- Dawes LG, Bowen C, Venta LA, et al. Ductography for nipple discharge: no replacement for ductal excision. Surgery 1998;124:685-91. [PubMed]
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg 2003;196:354-64. [PubMed]
- Moncrief RM, Nayar R, Diaz LK, et al. A comparison of ductoscopy-guided and conventional surgical excision in women with spontaneous nipple discharge. Ann Surg 2005;241:575-81. [PubMed]
- Liu GY, Lu JS, Shen KW, et al. Fiberoptic ductoscopy combined with cytology testing in the patients of spontaneous nipple discharge. Breast Cancer Res Treat 2008;108:271-7. [PubMed]
- Shen KW, Wu J, Lu JS, et al. Fiberoptic ductoscopy for patients with nipple discharge. Cancer 2000;89:1512-9. [PubMed]
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol 2007;14:3369-77. [PubMed]
- Dua RS, Isacke CM, Gui GP. The intraductal approach to breast cancer biomarker discovery. J Clin Oncol 2006;24:1209-16. [PubMed]
- Sabel MS, Helvie MA, Breslin T, et al. Is duct excision still necessary for all cases of suspicious nipple discharge? Breast J 2012;18:157-62. [PubMed]
- Valdes EK, Boolbol SK, Cohen JM, et al. Clinical Experience With Mammary Ductoscopy. Ann Surg Oncol 2006. [Epub ahead of print]. [PubMed]
- Sauter ER, Ehya H, Schlatter L, et al. Ductoscopic cytology to detect breast cancer. Cancer J 2004;10:33-41; discussion 15-6. [PubMed]
- Teboul M. Echo-histological “Acino-Ductal Analysis”. Preliminary results. Ultrasound Med Biol 1988;14 Suppl 1:89-95. [PubMed]
- Bender O, Balci FL, Yüney E, et al. Scarless endoscopic papillomectomy of the breast. Onkologie 2009;32:94-8. [PubMed]
- Hünerbein M, Dubowy A, Raubach M, et al. Gradient index ductoscopy and intraductal biopsy of intraductal breast lesions. Am J Surg 2007;194:511-4. [PubMed]
- Matsunaga T, Kawakami Y, Namba K, et al. Intraductal biopsy for diagnosis and treatment of intraductal lesions of the breast. Cancer 2004;101:2164-9. [PubMed]
- Tang SS, Twelves DJ, Isacke CM, et al. Mammary ductoscopy in the current management of breast disease. Surg Endosc 2011;25:1712-22. [PubMed]
- Balci FL, Feldman SM. Interventional ductoscopy for pathological nipple discharge. Ann Surg Oncol 2013;20:3352-4. [PubMed]
- Louie LD, Crowe JP, Dawson AE, et al. Identification of breast cancer in patients with pathologic nipple discharge: does ductoscopy predict malignancy? Am J Surg 2006;192:530-3. [PubMed]
- Jacobs VR, Paepke S, Schaaf H, et al. Autofluorescence ductoscopy: a new imaging technique for intraductal breast endoscopy. Clin Breast Cancer 2007;7:619-23. [PubMed]
- Feng XZ, Song YH, Zhang FX, et al. Diagnostic accuracy of fiberoptic ductoscopy plus in vivo iodine staining for intraductal proliferative lesions. Chin Med J (Engl) 2013;126:3124-9. [PubMed]
- Flanagan M, Love S, Hwang ES. Status of Intraductal Therapy for Ductal Carcinoma in Situ. Curr Breast Cancer Rep 2010;2:75-82. [PubMed]