
Pheochromocytoma/paraganglioma: recent updates in genetics, biochemistry, immunohistochemistry, metabolomics, imaging and therapeutic options
Introduction
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors (NETs) producing catecholamines and originating from adrenal medulla chromaffin cells or from neural crest cells outside the adrenal gland. In 80% of cases, these tumors arise in the adrenal medulla and in the remaining 20%, tumors arise outside the adrenal glands in the prevertebral and paravertebral sympathetic ganglia located mainly in the chest abdomen and pelvis (1,2). Using the latest WHO definitions, the term pheochromocytoma is used for tumors arising from the adrenal gland while tumors which arise outside the adrenal gland are termed as paraganglioma (3). Increased levels of catecholamines accounts for characteristic clinical manifestations particularly elevated blood pressure, headache, palpitations, and diaphoresis. PPGLs have an approximate incidence of 2–8 individuals per million population per year accounting for approximately 0.1% of individuals with hypertension (4). Most PPGLs are benign but there are a number of cases presenting initially as metastatic with an approximate incidence of one per million population per year. Patients with metastatic PPGL have a survival rate of 40–77% in five years and progression free survival ranges from 4–36 months (5). Metastatic PPGLs behave in a variable manner with some initially presenting with metastases and some developing metastases years after the initial diagnosis of PPGL. Factors correlated with an accelerated disease progression include male sex, diagnosis at an older age, synchronous metastases, bigger tumor size, increased dopamine level and failure to remove the primary tumor (6).
Genetics
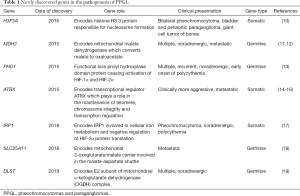
PPGLs have a high degree of heritability with 40% of cases carrying a germline mutation. Throughout the years, there has been more than 20 susceptibility genes identified. The underlying mutation influences PPGL clinical presentation such as cell differentiation, specific catecholamine production, tumor location, malignant potential and genetic anticipation (7-9) (Table 1). The Cancer Genome Atlas divided PPGL into 3 clinically useful molecularly defined groups: Kinase signaling subtype, Pseudohypoxia subtype, and Wnt-altered subtype (20).
Full table
Kinase signaling subtype
This subtype consists of somatic and germline mutations in NF1, RET, TMEM127 and HRAS genes (Table 1). Patients with these mutations typically present with pheochromocytoma having adrenergic biochemical phenotype corresponding to its high expression of phenylethanolamine N-methyltransferase (PNMT) that converts norepinephrine to epinephrine (20). Most of these pheochromocytomas are benign but have high degree of recurrence and multiplicity.
Pseudohypoxia subtype
Patients in this subtype present with both pheochromocytoma and paraganglioma and may produce either norepinephrine or dopamine or both. Thus, typically, epinephrine and metanephrine levels are within normal limits. This subtype is further divided into two main subgroups: tricarboxylic acid cycle (TCA)-related PPGLs that include mainly succinate dehydrogenase subunits A-D (SDHx), fumarate hydratase (FH), isocitrate dehydrogenase (IDH), and VHL/EPAS-related mutations. SDHx mutations are associated with PPGLs as well as gastrointestinal stromal tumors, pituitary adenomas, chondromas, neuroblastomas and very rarely gastroenteropancreatic tumors (21-24). SDHB mutation in particular was found to increase the risk for clinically aggressive PPGLs that are more likely to develop metastases or locally aggressive or recurrent tumors (7,25,26). In fact, SDHB mutation greatly affects outcome among patients with metastatic PPGLs exhibiting less disease-free interval and a shorter time interval between identification of the disease and first evidence of metastases (25,27). Interestingly, when compared to SDHD variant carriers who have a standard mortality ratio (SMR) of 0.93 which is comparable to the general population, SDHB variant carriers have a greater SMR at 1.89 which increases to 2.88 among SDHB variant carriers with a personal history of PPGL (28). Given its risk for metastases and association with poor outcomes, multiple studies have been done to determine the PPGL penetrance among individuals with an underlying SDHB mutation. A study showed that SDHB mutation has a PPGL penetrance of 49.80% at 85 years of age (29). Surprisingly, a difference in the age-related PPGL penetrance was noted between males and females with males having a 50% PPGL penetrance at age 74 but this was not reached in females (29). In addition, metastasis was noted in 85 out of 143 patients with PPGL (59.44%) with a median time of 3 years between initial diagnosis of PPGL and documentation of metastases. In another study, SDHB mutation was found to have a penetrance of 21% at 50 years of age but in contrast, there was no difference in the age-related PPGL penetrance between males and females (30). Benn et al. developed an approach to estimate lifetime disease penetrance of SDHx mutation by comparing allelic frequencies among individuals with and without PPGL (31). Using this approach, SDHB variants have an estimated lifetime disease penetrance of 22% as compared to SDHC and SDHA variants which have an estimated lifetime disease penetrance of 8.3% and 1.7% respectively.
FH mutation predisposes an individual to a syndrome of leiomyomatosis, renal cell carcinoma together with pheochromocytoma or paraganglioma (32-34). FH-related PPGLs are often metastatic or multiple. On the other hand, an EPAS1 mutation, also known as HIF2A mutation, results in a syndrome of multiple PPGLs, duodenal somatostatinomas and polycythemia also known as the Pacak-Zhuang syndrome with a high metastatic potential and multiplicity (35-38).
Wnt-altered subtype
This consists of adrenal pheochromocytomas associated with CSDE1 somatic mutations and MAML3 fusion genes activating the Wnt and Hedgehog signaling pathways (20). There are no known germline mutations in this subtype making it specific for sporadic pheochromocytoma. In patients with these mutations pheochromocytomas present as recurrent or metastatic.
Crona et al. looked into PPGLs in a PanCancer perspective (39). The PanCancer Initiative aims to ascertain similarities across various types of cancer and cell origin or within groups found to be associated based on anatomical or morphological characteristics (40). In the analysis of Crona et al., they found that PPGLs clustered with pancreatic NETs and neuroblastomas which challenges the current clinicopathological classification of PPGLs. PPGLs were found to share similarities with neuroblastomas, glioblastoma multiforme and brain lower grade glioma neuroblastoma. These similarities can be used to make careful conclusions about the developmental origin of these tumors or the role of microenvironment where these cancer cells develop from their precursors especially neural crest cells.
Furthermore, new susceptibility genes were identified during the past few years. Most of these newly discovered genes were identified using whole exome sequencing, some only in a few family members and some were together with other already known PPGL susceptibility genes. Moreover, using whole exome sequencing, 50% of apparently sporadic PPGLs were identified to have an underlying somatic mutation on the VHL, NF1 and RET genes (41). Identification of an underlying mutation whether germline or somatic, significantly influences current management and follow-up of PPGL. Furthermore, it serves as the platform in the discovery of innovative diagnostic and therapeutic options. Some new mutations associated with PPGL identified were not included in the Cancer Genome Atlas: H3F3A, MDH2, PHD1, IRP1, SLC25A11 and DLST. But it should be noted that some of these mutations were found in only a few family members or published as anecdotal case reports (10-13,17-19).
H3F3A gene
Chromatin-remodeling genes mutations indicates the presence of epigenetic modifications in PPGLs (42). H3F3A found in chromosome 1 encodes histone H3.3 protein responsible for nucleosome formation. In the study of Toledo et al., a patient carrying a H3F3A mutation presented with bilateral pheochromocytoma together with bladder and periaortic paragangliomas (10). This patient also has a history of recurrent tibial giant cell tumor which is similar to another patient carrying the same H3F3A mutation with an aggressive retroperitoneal paraganglioma with liver metastases and a history of recurrent and metastatic giant cell tumors.
MDH2 gene
A heterozygous variant on exon 4 of MDH2 was first detected in a patient with multiple metastatic paragangliomas (12). This gene was found to be responsible for encoding malate dehydrogenase enzyme that converts malate to oxaloacetate in the TCA cycle. The study demonstrated a lower MDH2 activity in mutated tumors but they were unable to document a subsequent accumulation of malate. However, a higher fumarate:succinate ratio was noted indicating fumarate accumulation likely explaining the PPGL development in this patient. Two of the index patient’s relatives were found to have the same mutation. Both of them were asymptomatic but one was identified to have the disease due to elevated levels of normetanephrine. MDH2 germline mutation is present in 0.6% of cases of PPGL with an incomplete penetrance (11).
PHD1 gene
PPGLs presenting with polycythemia has been first documented among patients harboring a mutation in PHD2, VHL, and HIF2A genes (38,43,44). Nevertheless, these mutations have been notably absent in a few patients who presented with the similar combination of PPGL and polycythemia, these findings were suggestive of other undiscovered mutations. Later on, in a patient who presented with pheochromocytoma accompanied by polycythemia without an underlying PHD2, VHL, and HIF2A mutations, Yang et al. found a new germline mutation in the PHD1 gene (13). This mutation resulted in the functional loss of prolyl hydroxylase domain protein thereby activating HIF-2α most likely contributing to pheochromocytoma tumorigenesis. Interestingly, there are some similarities between PPGLs secondary to HIF2A and PHD1 mutations. Both mutations manifest with multiple or recurrent PPGLs predominantly-secreting norepinephrine and presents with polycythemia at an early age. Despite both mutations presenting with polycythemia, erythropoietin is only significantly increased among patients carrying the HIF2A mutation in contrast to the mildly elevated level of erythropoietin brought about by the PHD1 mutation. Moreover, HIF2A mutation was found to be associated with duodenal somatostatinoma which has not been documented among patients with PHD1 mutation.
ATRX gene
ATRX gene located at chromosome X plays a role in the maintenance of telomeres, chromosome integrity and regulation of transcription (14). In a study by Fishbein et al., it was found that 12.6% of PPGLs carry somatic ATRX mutations and most of these patients also possess germline SDHx mutations (15). These patients demonstrated clinically aggressive behavior and alternative lengthening of telomeres. In contrast, a more recent study found that only 4% of PPGLs carry ATRX mutations (16). But similar to the previous study, half of these patients presented with clinically aggressive PPGLs and most of these patients had a concomitant SDHx mutation. This suggests that the identification of an underlying ATRX mutation can be used to assess the risk of PPGLs to demonstrate clinically aggressive behavior.
In most studies, ATRX mutations occur with another mutation in other known susceptibility genes such as SDHx but in a study by Comino-Méndez et al., a patient with an ATRX mutation without a coexisting mutation was also presented (45). That patient presented with pheochromocytoma with distant metastases. In the absence of another underlying mutation in the other known PPGL susceptibility genes, the authors concluded that the ATRX mutation was most likely the one that caused PPGL occurrence.
IRP1 gene
As described above, despite the identification of multiple genes associated with the syndrome of PPGL presenting with polycythemia, there are still some patients with a similar clinical presentation wherein no genetic mutation was identified. This led to the discovery of IRP1 by Pang et al. in a patient demonstrating polycythemia together with pheochromocytoma (17). IRP1 is a cellular iron metabolism regulator. In iron-deficient cells, IRP1 deletion causes a decrease in IRP1 protein levels leading to HIF-2α stabilization resulting in stimulation of EPO expression (46,47). In this case, Pang et al. were able to document a frame shift of exon 3 secondary to a splicing site mutation resulting in a heterozygous IRP1 deletion accounting for the clinical phenotype of polycythemia presenting with PPGL.
SLC25A11 gene
This tumor suppressor gene encodes the mitochondrial 2-oxoglutarate/malate carrier protein participating in the malate-aspartate shuttle. It is mainly involved in the transport of 2-oxoglutarate from the mitochondrial matrix to the cytoplasm via an electroneutral exchange with malate regenerating the mitochondrial NADH pool to facilitate the function of complex 1 of the electron transport chain (48). SLC25A11 mutation accounts for approximately 1% of PPGL cases (18). Furthermore, this mutation was associated with a malignant phenotype with 5% of all metastatic patients identified in a cohort of 121 patients have an underlying SLC25A11 mutation. Five out of seven patients identified to have SLC25A11 mutation were noted to have a malignant phenotype. It was concluded that this mutation should be considered as a new genetic risk factor which confers a predisposition to metastatic PPGL.
DLST gene
Dihydrolipoamide S-succinyltransferase (DLST) is another gene involved in the TCA which is believed to be one of the susceptibility genes for PPGLs. DLST is mainly involved in the E2 subunit of mitochondrial α-ketoglutarate dehydrogenase (OGDH) complex which facilitates conversion of α-ketoglutarate to succinyl-CoA and carbon dioxide. Remacha et al. detected this mutation in eight unrelated individuals accounting for 6% of patients with apparently sporadic PPGL (19). In terms of PPGL pathogenesis, this mutation is believed to cause the tumorigenesis by interfering with the normal function of the OGDH complex causing an increase in the levels of α-ketoglutarate which subsequently results to a higher α-ketoglutarate to fumarate ratio. It is worth mentioning that the identified individuals with the DLST mutation presented with multiple tumors. The identification of this mutation can greatly affect the treatment and monitoring plan for patients harboring DLST mutations.
Biochemistry
PPGLs secrete catecholamines which is responsible for the elevated blood pressure and other symptoms or signs such as palpitations, headaches and sweating which better characterize a patient with PPGL. Presenting symptoms of PPGL varies according to the specific catecholamine secreted by a tumor. Norepinephrine can act on both α- and β-adrenoceptors demonstrating a predominant effect on the α-adrenoceptors. α1-adrenoceptor activation causes vasoconstriction which results to elevation of blood pressure. Sustained hypertension may lead to cerebrovascular accidents, myocardial infarction, renal failure or intestinal ischemia (49). On the other hand, tumors mainly secreting epinephrine can present with hypotension because of β2-adrenoceptor activation causing vasodilation. This vasodilatory effect triggers reflex tachycardia which is typical among patients with an epinephrine-secreting PPGL (50). Dopamine-secreting tumors can also cause hypotension secondary to the vasodilatory effects of dopamine itself but this is very rare and only if plasma dopamine levels are very high. Dopamine-induced stimulation of D1 and D2 receptors may cause diarrhea, nausea and vomiting (51). In line with this, biochemical evaluation is an important step in the diagnosis of every PPGL. It is important to note that not all PPGLs produce and secrete catecholamines; there are tumors which do not produce catecholamines at all and are referred to as biochemically silent. Thus, PPGLs have been divided into four principal biochemical profiles which includes adrenergic, noradrenergic, dopaminergic, and silent (35).
In the past, biochemical evaluation was based on measurement of urine catecholamines and their metabolites such as vanillylmandelic acid and metanephrines (52). At present, it has been well documented from several recent studies that measurement of plasma free metanephrines is more reliable than plasma catecholamines, urinary catecholamines, urinary total metanephrines, urinary vanillylmandelic acid and plasma deconjugated metanephrines (52-55) (Table 2). Particularly, in a recent study by Eisenhofer et al. (58), it was found that the measurement of plasma free metanephrines has a higher sensitivity and specificity (96.6% and 94.9%, respectively), compared to measurement of urinary free metanephrines (92.9% and 94.5%, respectively). They also found a significant difference in the sensitivity and specificity of both tests when the patients being tested were classified according to the risk of developing a PPGL. A high risk of developing PPGL was defined by the presence of an adrenal incidentaloma, previous history of PPGL or an underlying mutation in PPGL susceptibility genes. The measurement of plasma free metanephrines was found to be a better screening tool for PPGL than the measurement of urinary fractionated metanephrines among patients with a high risk of having PPGLs with a sensitivity of 96.7% and specificity of 92.8%. As for patients who have a low risk of having PPGL meaning they were tested on the basis of presence of signs and symptoms suggestive of disease without any history of previous disease, adrenal incidentaloma or known susceptibility gene mutation, plasma or urinary fractionated metabolites can be used as a screening tool without significant difference in their performance. It is important to note that the superiority of plasma free metanephrines will only hold true if an accurate method of measurement, technique and established reference intervals are employed. The US Endocrine Society guidelines recommend the use of liquid-chromatography with electrochemical detection (LC-ECD) or liquid-chromatography with tandem mass spectrometry (LC-MS/MS) in the biochemical evaluation of PPGLs. In addition, it is important to note that measurements of plasma free metanephrines should be facilitated with the patient in a supine position for at least 30 min (59). An upright position may falsely elevate plasma metanephrines level secondary to an increase in norepinephrine/epinephrine release brought about by sympathoadrenal activation. Of note, most laboratory reference values are determined from measurements taken in the upright or seated position. In line with this, laboratories are advised to establish their reference values from data taken in the supine position (60). It is also recognized that age affects the levels of catecholamines and metanephrines. Therefore, it is suggested that age-appropriate levels should be used particularly when the patient belongs in the pediatric age group (61).

Full table
The process by which we measure levels of catecholamine and its metabolites has also changed throughout the years. Traditional bioassays, colorimetric, fluorometric techniques and radioenzymatic assays were used in the past for the biochemical diagnosis of PPGL but our technology have progressed to the use of LC-ECD or LC-MS/MS. With the availability of these more advanced techniques, measurement of fractionated metanephrines was made possible which is an important improvement over measurements of total metanephrines using the colorimetric or fluorometric technique (61). The level of plasma metanephrines using enzyme immunoassay was found to be suboptimal compared to levels measured by LC-MS/MS (56,62,63). A higher sensitivity and specificity for the diagnosis of PPGLs were also noted using LC-MS/MS making it the current method of choice for plasma free metanephrines measurement (56,63). In addition, the use of LC-ECD and LC-MS/MS enabled the measurement of methoxytyramine levels which is a dopamine metabolite (61). Measurement of methoxytyramine in addition to the standard panel of plasma free metanephrines has been shown to increase the detection of PPGLs modestly because it allows the identification of tumors that exclusively produce dopamine. In addition to metanephrine and normetanephrine, methoxytyramine measurement significantly increased the diagnostic sensitivity for head and neck paragangliomas (HNPGLs) from 22.1% to 50% (57). This signifies the importance of determining methoxytyramine levels among patients suspected or with known HNPGL. One of the difficulties encountered in the biochemical diagnosis of PPGLs is the interpretation of results under the influence of medications which can interfere with analysis (60,64). A number of patients who would require biochemical testing are usually on antihypertensive medications which can also affect analysis. The use of LC-ECD was found to be susceptible to analytical interference with the use of several medications including antihypertensive drugs such as phenoxybenzamine and α-adrenoceptor blockers (65-67). One of the advantages of using LC-MS/MS is its lack of interference from antihypertensive drugs such as α-adrenoceptor blockers, diuretics and ACE inhibitors (68). Using this technique in the measurement of plasma metanephrines enables the patients to continue their current antihypertensive regimen decreasing the risk of sudden elevations of blood pressure. In addition, the use of mass spectrometry also provides easier workflows, short chromatography run-times and higher throughput (69).
As mentioned earlier, the excess levels of catecholamines among patients with PPGLs may cause hypertensive crisis and in severe cases may also cause end organ damage in the form of myocardial infarction, cerebrovascular accident or multiple organ failure especially during stressful conditions particularly while ongoing a surgical procedure. PPGLs possess an excessive amount of catecholamines and its release may be elicited by induction of general anesthesia, abdominal pressure fluctuation as well as direct tumor handling during the procedure itself. With the increased risk for adverse cardiovascular events, preoperative blockade using α- and β-adrenoceptor blockers and calcium channel blockers is very highly recommended (60,70,71). Preoperative adrenoceptor blockade has been shown to prevent complications such as major adverse cardiovascular events pre- as well as peri-operatively (72). Although current recommendations by the US Endocrine Society guidelines strongly advocate the use of preoperative blockade (60), there are other groups that question the use of pre-operative adrenoceptor blockade because they were unable to document a significant difference in the incidence of hypertensive episodes and major complications between patients who underwent preoperative blockade and those who did not (73). A recent review has also been released which states that there is a low risk of decompensation prior to surgery for patients not on pre-operative blockade without documented bad outcomes despite intermittent blood pressure elevations. They further stated that given this data, surgical treatment may be facilitated without delay which is sometimes secondary to the need to initiate preoperative adrenoceptor blockade (74). This approach is considered as erroneous by our group and at least in US could lead to very complicated situations like when a patient deteriorates shortly before an operation (e.g., sudden hypertensive crisis or tachyarrhythmia resulting in stroke or other complications) or when a physician could be investigated for inappropriate clinical care based on current US guidelines and recommendations (60,70,71,74). This scenario is well summarized by Wolf et al. (75) who pointed out that unexpected situations do occur not only during surgery but before as well, which may significantly increase the probability of an adverse event, hence, there is a need for physicians to be always aware of the risks involved and safeguard patients from these catastrophic events. In addition, our group recognizes well the risk of potential unforeseen adverse events among patients with PPGL and if this adverse outcome indeed happens without the use of preoperative adrenoceptor blockade, physicians may be at risk for ethical issues and may even be accused of medical malpractice or negligence.
Immunohistochemistry
Despite the availability of genetic testing to identify an underlying driver mutation for individuals with PPGL, it is laborious and expensive, especially in some countries. To resolve this issue, immunohistochemistry can be used as a guide to limit time and expenses required to identify the underlying mutation (76). In addition, immunohistochemistry can also be used to assess the pathogenicity of variants of uncertain significance identified using genetic testing (77) (Table 3).

Full table
Immunohistochemistry identifies SDHx mutations by loss of SDHB or SDHA protein expression. Among individuals with SDHB, SDHC, and SDHD mutations, their tumors are negative for SDHB immunostaining and positive for SDHA immunostaining in contrast to individuals with SDHA mutation only who are negative for both SDHB and SDHA immunostaining (77,79,82) (Table 3). SDHB immunohistochemistry have a strong correlation with an underlying SDHx subunit gene mutation with a sensitivity and specificity of 95.0% and 81.8%, respectively (79). Interobserver variation analysis using SDHB/SDHA immunohistochemistry was assessed and it was noted that sensitivity for this approach ranged from 83.58% to 98.57% with a mean of 94.23%; specificity ranged from 74.03% to 96.11% with a mean of 84.35% (77). Although SDHB/SDHA immunohistochemistry is a useful tool in the identification of PPGLs associated with a SDHx mutation, there are instances when there is disagreement in the interpretation of a weakly diffuse SDHB immunostaining. This weak diffuse signal is usually observed among patients with SDHD mutations (83). Tumors with an underlying SDHD mutation present with a diffuse cytoplasmic blush which could be mistakenly read as a granular cytoplasmic staining characteristic of a non-SDHx related tumor (84). When a weak diffuse SDHB immunostaining is erroneously read as positive, we miss identifying an individual with a SDHx mutation compromising patient care. To resolve this issue, SDHD immunostaining was proposed as a complementary tool. Interestingly, patients with SDHx related PPGLs are positive on SDHD immunostaining as compared to non-SDHx related tumors that are negative on SDHD immunostaining (78). The SDH complex has a catalytic and anchoring component. SDHA and SDHB forms the catalytic component while SDHC and SDHD functions as an anchor which connects the complex to the inner mitochondrial membrane (84). It is hypothesized that because SDHB and SDHA protein levels are also low in non-SDHx-related PPGLs, SDHC and SDHD protein levels are low too causing the SDHD protein to be masked, thereby, demonstrating a negative SDHD immunostaining. On the other hand, among SDHx-related tumors, there is disruption of the active complex that uncovers SDHD protein resulting in a positive SDHD immunostaining. This finding is particularly useful in verifying possible false positives in tumors presenting with a weak diffuse SDHB immunostaining. A negative SDHD immunostaining together with a weak diffuse SDHB immunostaining indicates a non-SDHx related tumor while a positive SDHD immunostaining in the presence of a weak diffuse SDHB immunostaining is indicative of a SDHx-related tumor (78).
As mentioned, FH mutation leads to a syndrome of hereditary leiomyomatosis and renal cell carcinoma together with pheochromocytoma or paraganglioma. It has been shown that leiomyomas and renal cell cancer with FH mutation leads to a loss of f FH protein expression (34). Given that there is only a small subset of patients with PPGL with an underlying FH mutation, studies on immunohistochemistry of these tumors are limited. In a study by Udager et al., a patient with retroperitoneal mass with an underlying FH mutation revealed a loss of cytoplasmic FH immunohistochemistry staining (79).
Carbonic anhydrase 9 (CA9) preserves normal intracellular and extracellular pH by stimulating carbon dioxide hydration. It is recognized as a hypoxia-induced gene and was found to be increased in renal clear cell carcinoma which is associated with a malfunctioning VHL-hypoxia inducible factor pathway (85). In line with this, CA9 is strongly expressed in pheochromocytomas with an underlying VHL mutation (86). Favier et al. were able to demonstrate a positive CA9 immunostaining for 42 out of 48 (88%) patients with VHL mutation (81). Immunostaining for CA9 was also found to be negative for 144 out of 159 (91%) patients without a VHL mutation. They proposed that CA9 immunohistochemistry could be tested in all PPGLs to identify underlying germline or possibly somatic VHL mutation. This technique can also be used to assess the pathogenicity of VHL variants of uncertain significance identified using genetic testing.
It is known that PPGLs are made of chromaffin cells. Sustentacular cells which are stained using S100 protein are almost always present among PPGLs. In a more recent study, it has been found that in addition to sustentacular cells, CD163 and CD68 positive cells were identified indicating the presence of cells with monocyte-macrophage lineage but they were unable to find a significant association of monocyte to sustentacular cell ratio and genotype or location of tumor. Despite this lack of association, identification of these monocytes within PPGLs can serve as a catalysis for the development of new treatment modalities (80).
Metabolomics
According to the Warburg hypothesis, cancer is caused by an impairment in the mitochondrial respiratory function causing a shift from oxidative phosphorylation to glycolysis. A number of signaling and metabolic pathways are activated secondary to this cellular respiration shift which promotes proliferation and prolongs survival of cells (87). As described above, some cases of PPGLs are known to be caused by mutations in genes encoding proteins involved in the Krebs cycle. Mutation in the genes encoding SDHx, FH, MDH2, and IDH predispose an individual to develop hereditary PPGLs. A pathogenic mutation in one of these gene leads to accumulation of succinate, fumarate, or 2-hydroxyglutarate which causes α-ketoglutarate dependent enzyme inhibition resulting to pseudohypoxia and hypermethylation which is responsible for tumorigenesis (88). Using new technologies, nuclear magnetic resonance spectroscopy and gas chromatography-mass spectrometry or liquid chromatography-mass spectrometry can now be used to profile and quantify the resulting metabolite accumulation brought by these pathogenic mutations (89). This is especially important in any situation when genetic testing yields unresolved results wherein in a variant of uncertain significance may be identified. In these instances, metabolomic data of tumor tissue can help verify functionality of the underlying germline or somatic variants. Thus, identification and quantification of metabolites accumulated in a tumor can be used to uncover new PPGL susceptibility genes which in turn could serve as a new platform for novel diagnostic and therapeutic options. Accumulation of certain metabolites can also be used as a marker for metastases and monitoring of therapeutic response (90-92) (Table 4).

Full table
SDHx mutations result in succinate accumulation. The resulting higher succinate:fumarate ratio enables differentiation from non-SDHx PGLs making it a very useful metabolic marker (90,94,95). Tumor tissue succinate:fumarate ratios can recognize SDHx-related PPGLs with a sensitivity and specificity of 93% and 97%, respectively which is similar to the performance of other available diagnostic tests (92). Interestingly, quantification of succinate as a screening test for SDHx-related PPGLs resulted in 100% sensitivity and specificity using a threshold of 0.253 nmol/mg to distinguish them from non-SDHx PPGLs and 0.096 nmol/mg to differentiate them form sporadic PPGLs (93).
Succinate:fumarate ratio was also found to be higher in metastatic disease as compared to non-metastatic disease (92). This highlights the potential of succinate:fumarate ratio as a marker for metastatic disease. Furthermore, the measurement of succinate:fumarate ratio, especially in plasma, could also be used to monitor PPGL patients and their therapeutic response including any recurrence or new metastasis (90).
Imaging
The accessibility of new functional imaging modalities have added value in accurate diagnosis of PPGLs (96). As recommended by the US Endocrine Society Guidelines, a confirmatory biochemical evidence of disease is required before contemplating on any imaging tool in PPGLs (60). Computed tomography (CT) and magnetic resonance imaging (MRI) are the recommended initial anatomic imaging modalities after biochemical confirmation (96,97). After lesion determination using CT or MRI, the decision whether additional whole-body anatomic and functional studies are necessary depends on the biochemical profile, tumor size, as well as likelihood for metastasis. Patients who present with extra-adrenally located lesions greater than 5 cm, secreting norepinephrine/normetanephrine or dopamine/methoxytyramine, will more likely benefit from such imaging to further define extent of disease due to a higher metastatic potential (97). All patients with SDHB-related PPGL and those in whom surgical specimen of tumor tissue detected ATRX and HIF2A mutations and primary multiple PPGLs, will also benefit from those studies (39,98).
Iodine-123-metaiodobenzylguanidine (123I-MIBG) scintigraphy
With regards to the functional imaging approaches, 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy is currently being utilized for imaging of pheochromocytomas believed to be sporadic (99). Aside from being extensively available and relatively inexpensive, high-quality images and relatively low radiation exposure are some of its advantages (100). Abnormal findings on 123I-MIBG scintigraphy include a noticeable increased adrenal uptake with concomitant gland enlargement as well as focal uptakes outside the adrenal tissue without normal physiologic distribution (101) (Table 5). A number of studies on pheochromocytoma patients have documented sensitivity and specificity of 123I-MIBG scintigraphy at 83–100% and 95–100%, respectively. However, lower sensitivity of 52–75% was found in patients having extra-adrenal, multiple, recurrent, and hereditary PPGLs. Nonetheless, it is of great use in metastatic disease when radiotherapy using 131I-MIBG is planned or when there is an increased risk of metastasis and recurrence due to a large size of primary or extra-adrenal tumor (96,99).
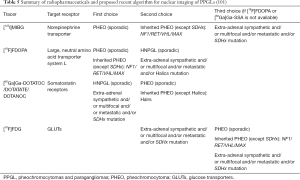
Full table
Expression of several transporters and receptors by PPGLs such as norepinephrine transporter, glucose transporter (GLUT), amino acid transporter, and somatostatin receptor (SSTR) has given much attention to novel nuclear imaging modalities targeting such receptors (Table 5). The following positron emission tomography hybridized with CT (PET/CT) radiopharmaceuticals are presently being employed in disease evaluation, treatment plan, and assessment of tumor response: 18F-fluorodeoxyglucose (18F-FDG), 18F-fluorodopa (18F-FDOPA), and 68Gallium (68Ga)- tetraazacyclododecanetetraacetic acid (DOTA) analogs (96,101).
68Gallium PET/CT SSTR analogues
PET/CT imaging with 68Ga-labeled DOTA peptides such as DOTA(0)-Tyr(3)-octreotate (DOTATATE), DOTA(0)-Phe(1)-Tyr(3)-octreotide (DOTATOC), and DOTA(1)-Nal(3)-octreotide (DOTANOC) bind to SSTR-expressing tumors more efficiently and has quickly evolved as the favored functional imaging modality for PPGLs in general (97,101). More specifically, DOTATATE has greater affinity for SSTR2 receptors. In a recent study by Archier et al., a higher sensitivity of 93% for 68Ga-DOTATATE PET/CT compared to 18FDOPA PET/CT (89%), and conventional imaging (76%) was observed in terms of lesion detection and localization. This has been mainly credited to its high tumor-to-background uptake ratio (102). Moreover, 68Ga-DOTATATE PET/CT have shown superiority over other functional imaging scans in improved detection of sporadic, SDHB-metastatic disease, as well as primary and SDHD-related HNPGLs (97,102-104). Aside from its significant role in primary tumor detection, it can further identify patients eligible for peptide receptor radionuclide therapy (PRRT) with radiolabeled SSTR agonists (105).
SSTR antagonists
One of the latest developments concerning SSTR targeting is the introduction of SSTR antagonists (111In-DOTA-BASS, 111In-DOTA-JR11, Ga-DOTA-JR11, Ga-NODAGA-JR11). Preclinical and clinical studies at present have shown evidence of greater tumor uptake, despite lack of internalization, which may improve image quality and increase lesion detection by SSTR PET/CT (101,105). When compared to an SSTR agonist in the use of human specimens, 125I-JR11 (an SSTR antagonist) have shown increased avidity to SSTR2 sites resulting to better tumor localization and value of treatment approach using radiolabeled SSTR antagonists. Aside from pheochromocytoma, other tumors that can be explored by imaging using SSTR2 antagonists are breast cancer, small cell lung cancer, renal cell cancer, non-Hodgkin’s lymphomas, and medullary thyroid cancer (105).
18F-fluorodopa PET/CT
The application of 18F-FDOPA PET/CT in the imaging of L-type amino acid transporters (mainly LAT1 and LAT2) it a well-accepted imaging tool to spot pheochromocytomas (101). It has a sensitivity of up to 100% for primary pheochromocytomas equivocal on 123I-MIBG scintigraphy (106). Also, it is an excellent diagnostic tool for HNPGLs, but with lower sensitivity for retroperitoneal paragangliomas related to SDHx mutations. In terms of metastatic disease, it offers higher lesion detection rate in SDHB-negative patients than in SDHB-positive ones (100).
Whereas 68Ga-DOTATATE PET/CT do better than other imaging techniques in the evaluation of PPGLs, its high uptake by the healthy adrenal glands poses a problem in the discovery of small pheochromocytomas associated. On the other hand, 18F-FDOPA has the advantage of detecting these small lesions related to gene mutations that result in multiple endocrine neoplasia type 2 (MEN2), neurofibromatosis type 1 (NF1), von Hippel-Lindau (VHL) and other paraganglioma (PGL) syndromes due to its very low physiologic uptake in the normal adrenal medulla (101,102). This imaging modality also helps in the assessment of patients with MAX mutations developing bilateral/multiple tumors in the same gland (36). Moreover, it was found to surpass 68Ga-DOTATATE in identifying other primary tumors and/or metastases in somatic HIF2A mutation patients. Furthermore, 18F-FDOPA can be utilized for HNPGL detection in the absence of 68Ga-DOTATATE with a sensitivity and specificity of >90% and 95–100% respectively (36).
18F-Fluorodeoxyglucose PET/CT
18F-FDG PET/CT is a widely accepted imaging tool in oncology from disease diagnosis to monitoring of treatment response. It has an estimated overall average sensitivity and specificity of 84% and 88% respectively, in cancer (107). Aside from it being generally available, nuclear imaging with 18F-FDG PET/CT is preferred in an established metastatic disease. It has also been suggested for the localization and prognosis of highly aggressive NETs and non-NETs (106). In particular, its acceptable sensitivity in metastatic PGLs mainly related to SDHx mutation has been well recognized which is in contrary to 18F-FDOPA PET/CT (96,100,101,103,106). Though 123I-MIBG scintigraphy is a radiotherapeutic option for metastatic PPGLs, unimpressive detection rates when it comes to tumors related to SDHB has led to an enhanced application of 18F-FDG PET/CT and other newly developed functional imaging in the evaluation of biochemically proven hereditary PPGLs (108,109). Currently, 18F-FDG PET/CT present a reasonable and readily accessible imaging option for patients with SDHx-related tumors, extra-adrenal PPGLs, with multifocality/metastases (101,109).
Treatment
There has been no standardized protocol in the treatment of metastatic PPGL and that prospective studies are lacking due to its rarity. Despite ongoing developments in treatment, factors determining prognosis and survival are yet to be defined (98). Genetic predilection, tumor load, biochemical phenotype, disease progression, and presence of metastases primarily determine disease prognosis (110). More specifically, better prognosis was observed in the following variables: young age, female gender, aortic/carotid body tumors, complete surgical resection, local disease, and presence of second primary malignancy (98). Moreover, better survival was associated with HNPGLs, metanephrines less than five-fold the upper limits of normal range, and low proliferative index (5). On the other hand, reduced survival rate and poor prognosis were more correlated to male sex, older age at time of diagnosis, larger primary tumor size, high dopamine levels, incomplete resection, and presence of distant metastasis (6,111). Both localized (radiotherapy, radiofrequency, or cryoablation) and systemic therapies (chemotherapy or molecular targeted therapies) are available therapeutic options when surgery is not generally feasible (110). Specifically, in terms of localized treatment approach, ablative therapy in metastatic PPGL can successfully result in palliation of symptoms and decrease in tumor burden (112). Currently, there has been an increasing interest on the value of radionuclide therapy for NETs - 131I-MIBG and the very recent PRRT (110).
Radionuclide therapy implicates the use of radiolabeled peptides with high affinity to the involved receptor. In addition to surgery and chemotherapy, 131I-MIBG treatment has been effectively used in the management of malignant and metastatic PPGLs. 177Lu-DOTATATE (Lutathera®) therapy has been recently used in metastatic PPGLs (113,114).
131I-MIBG therapy
Treatment with high-specific activity 131I-MIBG (Azedra®) has been very recently being employed to treat patients with metastatic PPGLs including those with unsatisfactory outcomes even after conventional therapies (115,116). The beta particles from this radionuclide are responsible for damaging the cells while the gamma ray permits dosimetry calculations. Using the high specific activity formulation (2,500 mCi/mg) allows better targeting and higher tumor concentration (117). It has become the first systemic radiotherapy for metastatic PPGLs approved by United Stated Food and Drug Administration (FDA) in July 2018 (115). In a study by Gonias et al., overall response and survival rates of 57% and 64% respectively. Furthermore, SDHB patients were observed to achieve complete/partial response more likely and that prior chemotherapy or radiation were poor predictors of survival (118). Data from a pivotal phase II open-label, multicenter trial involving patients with MIBG-positive metastatic PPGLs showed that within 12 months after treatment with Azedra, prolonged blood pressure control with partial response and stable disease in 92% of patients were significantly observed (116). The most common observed adverse effects were myelosuppression, nausea, vomiting, fatigue and dizziness (106,115).
Peptide receptor radionuclide therapy (PRRT)
177Lu-DOTATATE (Lutathera®) has been recently approved in the United States for treatment of NETs (106). A recent retrospective study by Vyakaranam et al. presented positive outcomes of 177Lu-DOTATATE as first-line therapy or for progressive PPGL, after showing evidence of increased overall survival with better scintigraphic (>50%) and biochemical responses (>50% decrease) (119). Moreover, in a study of 20 PPGL patients by Kong et al., partial and stable disease was noted in 29% and 62% of patients respectively, 3 months after 177Lu-DOTATATE treatment (113). In a study by Yadav et al., capecitabine with concomitant 177Lu-DOTATATE treatment in malignant PGL achieved partial tumor response and stable disease in 28% and 56% of patients respectively. A decrease in chromogranin A levels was also observed in 28% of patients (114). Despite evidence of treatment response, modified protocols using longer radioisotope infusion time and lesser administered dose are being utilized to prevent severe adverse reactions such as catecholamine crisis and tumor lysis syndrome (120).
Chemotherapy
An existing management for advanced PPGLs includes chemotherapy with combination regimen of cyclophosphamide/vincristine/dacarbazine (CVD). In 50% of cases, CVD therapy causes relief of symptoms and tumor regression but only temporary (121). Temozolomide (TMZ), a novel alkylating agent, has been utilized as an alternative chemotherapeutic agent to dacarbazine in various types of tumor (121). Moreover, it has been proposed to be more efficient in SDHB-related metastatic PPGLs, inferring that PPGLs are effectively targeted depending on genetic background (122). Poly (ADP-ribose) polymerase (PARP) is an enzyme which prevents DNA breakage and instability by producing ADP-ribose–conjugated PARP (PADPR). Since most chemotherapeutic agents including TMZ initiate DNA-damaging effects, combination with PARP inhibitors can be an effective treatment strategy for metastatic tumors (121). Findings in a recent experimental study by Pang et al. have shown improved therapeutic effects of genotoxic agents, lesion reduction, and prolonged overall survival by combining PARP inhibitor olaparib with TMZ targeting the nicotinamide adenine dinucleotide (NAD+)/PARP DNA repair pathway in SDHB-mutated experimental pheochromocytoma cells (121).
BEZ235 is another chemotherapeutic agent recently being studied for treatment of malignant pheochromocytoma. It generates anti-tumoral effects by inhibiting both phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin complex 1 or 2 (mTORC1/2) kinase activity through attachment to the ATP-binding part of these enzymes (123,124). In vivo study on rat pheochromocytomas by Lee et al. have shown that treatment with BEZ235 resulted to a decline in norepinephrine transporter expression by inducing cytotoxic and anti-proliferative effects (125). Altogether, PI3K/mTOR inhibition has demonstrated induction of cell apoptosis with significant reduction in cell proliferation and angiogenesis contributing to the response of experimental pheochromocytoma cells (125).
223Radium dichloride (Xofigo)
Radium chloride (Ra) for treatment of patients with castration resistant metastatic prostate cancer became available in 2013 (126). Patients who may benefit from 223Ra-chloride are selected through bone scintigraphy and the activity administered is calculated based on the patient’s weight and not the degree of accumulation of activity on the diagnostic scan (126). In a case report by Makis et al. in 2016, 223Ra treatment in a patient with SDHB positive malignant hereditary PPGL syndrome improved pain control and ambulation (127). The primary treatment-related adverse event is myelosuppression including anemia, thrombocytopenia, and neutropenia (128). However, future prospective studies are yet to be needed to establish its efficacy and therapeutic benefits in metastatic PPGL bone metastases.
Other therapies including immunotherapy
In cases of malignant pheochromocytomas refractory to both radiation therapy and chemotherapy, tyrosine kinase inhibitors such as Sunitinib, appear to be beneficial. Sunitinib, FDA-approved for the treatment of pancreatic NETs, displays both antiproliferative and antitumor effects through suppression of tumor angiogenesis by inhibiting vascular endothelial growth factor (VEGF) as well as direct inhibition of catecholamine synthesis and secretion (129). In a retrospective study involving metastatic PPGL patients, 47% demonstrated partial response, stable disease, with improvement in blood pressure and performance status after Sunitinib treatment (130). Furthermore, in a recent study using immunotherapy in a pheochromocytoma mouse model, intratumoral injections of mannan-BAM, toll-like receptor ligands, and anti-CD40 led to tumor volume stabilization, reduction in liver metastatic lesions, and longer median survival (131).
Until now, inadequate treatment options are available for metastatic PPGLs. Chemotherapy and radionuclide therapy as conventional therapeutic approach offer symptom relief and biochemical control however achieve less in terms of increasing survival. In the future, novel treatment strategies might be more effective recognizing the value of specific signaling pathways and molecular targets responsible for the development of malignant PPGL.
Acknowledgments
Funding: This article was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gupta G, Pacak K, Committee AAS. Precision Medicine: An Update on Genotype/Biochemical Phenotype Relationships in Pheochromocytoma/Paraganglioma Patients. Endocr Pract 2017;23:690-704. [Crossref] [PubMed]
- Pang Y, Liu Y, Pacak K, et al. Pheochromocytomas and Paragangliomas: From Genetic Diversity to Targeted Therapies. Cancers (Basel) 2019. [Crossref] [PubMed]
- Lloyd RV, Osamura RY, Klöppel G, et al. WHO classification of tumours of endocrine organs. 4th edition. World Health Organization Classification of Tumours, vol tenth volume. Lyon: International Agency for Research on Cancer (IARC), 2017.
- Kopetschke R, Slisko M, Kilisli A, et al. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 2009;161:355-61. [Crossref] [PubMed]
- Hescot S, Curras-Freixes M, Deutschbein T, et al. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J Clin Endocrinol Metab 2019;104:2367-74. [Crossref] [PubMed]
- Hamidi O, Young WF Jr, Iniguez-Ariza NM, et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab 2017;102:3296-305. [Crossref] [PubMed]
- Crona J, Lamarca A, Ghosal S, et al. Genotype-phenotype correlations in pheochromocytoma and paraganglioma. Endocr Relat Cancer 2019. [Crossref] [PubMed]
- Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer 2014;14:108-19. [Crossref] [PubMed]
- Jochmanova I, Pacak K. Genomic Landscape of Pheochromocytoma and Paraganglioma. Trends Cancer 2018;4:6-9. [Crossref] [PubMed]
- Toledo RA, Qin Y, Cheng ZM, et al. Recurrent Mutations of Chromatin-Remodeling Genes and Kinase Receptors in Pheochromocytomas and Paragangliomas. Clin Cancer Res 2016;22:2301-10. [Crossref] [PubMed]
- Calsina B, Curras-Freixes M, Buffet A, et al. Role of MDH2 pathogenic variant in pheochromocytoma and paraganglioma patients. Genet Med 2018;20:1652-62. [Crossref] [PubMed]
- Cascón A, Comino-Mendez I, Curras-Freixes M, et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst 2015. [Crossref] [PubMed]
- Yang C, Zhuang Z, Fliedner SM, et al. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J Mol Med (Berl) 2015;93:93-104. [Crossref] [PubMed]
- Clynes D, Gibbons RJ. ATRX and the replication of structured DNA. Curr Opin Genet Dev 2013;23:289-94. [Crossref] [PubMed]
- Fishbein L, Khare S, Wubbenhorst B, et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun 2015;6:6140. [Crossref] [PubMed]
- Job S, Draskovic I, Burnichon N, et al. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res 2019;25:760-70. [Crossref] [PubMed]
- Pang Y, Gupta G, Yang C, et al. A novel splicing site IRP1 somatic mutation in a patient with pheochromocytoma and JAK2(V617F) positive polycythemia vera: a case report. BMC Cancer 2018;18:286. [Crossref] [PubMed]
- Buffet A, Morin A, Castro-Vega LJ, et al. Germline Mutations in the Mitochondrial 2-Oxoglutarate/Malate Carrier SLC25A11 Gene Confer a Predisposition to Metastatic Paragangliomas. Cancer Res 2018;78:1914-22. [Crossref] [PubMed]
- Remacha L, Pirman D, Mahoney CE, et al. Recurrent Germline DLST Mutations in Individuals with Multiple Pheochromocytomas and Paragangliomas. Am J Hum Genet 2019;104:1008-10. [Crossref] [PubMed]
- Fishbein L, Leshchiner I, Walter V, et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017;31:181-93. [Crossref] [PubMed]
- Xekouki P, Pacak K, Almeida M, et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab 2012;97:E357-66. [Crossref] [PubMed]
- Settas N, Faucz FR, Stratakis CA. Succinate dehydrogenase (SDH) deficiency, Carney triad and the epigenome. Mol Cell Endocrinol 2018;469:107-11. [Crossref] [PubMed]
- Schimke RN, Collins DL, Stolle CA. Paraganglioma, neuroblastoma, and a SDHB mutation: Resolution of a 30-year-old mystery. Am J Med Genet A 2010;152A:1531-5. [PubMed]
- Niemeijer ND, Papathomas TG, Korpershoek E, et al. Succinate Dehydrogenase (SDH)-Deficient Pancreatic Neuroendocrine Tumor Expands the SDH-Related Tumor Spectrum. J Clin Endocrinol Metab 2015;100:E1386-93. [Crossref] [PubMed]
- Assadipour Y, Sadowski SM, Alimchandani M, et al. SDHB mutation status and tumor size but not tumor grade are important predictors of clinical outcome in pheochromocytoma and abdominal paraganglioma. Surgery 2017;161:230-9. [Crossref] [PubMed]
- Turkova H, Prodanov T, Maly M, et al. Characteristics and Outcomes of Metastatic Sdhb and Sporadic Pheochromocytoma/Paraganglioma: An National Institutes of Health Study. Endocr Pract 2016;22:302-14. [Crossref] [PubMed]
- Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab 2007;92:3822-8. [Crossref] [PubMed]
- Rijken JA, van Hulsteijn LT, Dekkers OM, et al. Increased Mortality in SDHB but Not in SDHD Pathogenic Variant Carriers. Cancers (Basel) 2019. [Crossref] [PubMed]
- Jochmanova I, Wolf KI, King KS, et al. SDHB-related pheochromocytoma and paraganglioma penetrance and genotype-phenotype correlations. J Cancer Res Clin Oncol 2017;143:1421-35. [Crossref] [PubMed]
- Rijken JA, Niemeijer ND, Jonker MA, et al. The penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin Genet 2018;93:60-6. [Crossref] [PubMed]
- Benn DE, Zhu Y, Andrews KA, et al. Bayesian approach to determining penetrance of pathogenic SDH variants. J Med Genet 2018;55:729-34. [Crossref] [PubMed]
- Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet 2014;23:2440-6. [Crossref] [PubMed]
- Clark GR, Sciacovelli M, Gaude E, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab 2014;99:E2046-50. [Crossref] [PubMed]
- Skala SL, Dhanasekaran SM, Mehra R. Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome (HLRCC): A Contemporary Review and Practical Discussion of the Differential Diagnosis for HLRCC-Associated Renal Cell Carcinoma. Arch Pathol Lab Med 2018;142:1202-15. [Crossref] [PubMed]
- Crona J, Taieb D, Pacak K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr Rev 2017;38:489-515. [Crossref] [PubMed]
- Därr R, Nambuba J, Del Rivero J, et al. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer 2016;23:899-908. [Crossref] [PubMed]
- Pacak K, Jochmanova I, Prodanov T, et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol 2013;31:1690-8. [Crossref] [PubMed]
- Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 2012;367:922-30. [Crossref] [PubMed]
- Crona J, Backman S, Welin S, et al. RNA-Sequencing Analysis of Adrenocortical Carcinoma, Pheochromocytoma and Paraganglioma from a Pan-Cancer Perspective. Cancers (Basel) 2018. [Crossref] [PubMed]
- Cooper LA, Demicco EG, Saltz JH, et al. PanCancer insights from The Cancer Genome Atlas: the pathologist's perspective. J Pathol 2018;244:512-24. [Crossref] [PubMed]
- Urbini M, Nannini M, Astolfi A, et al. Whole Exome Sequencing Uncovers Germline Variants of Cancer-Related Genes in Sporadic Pheochromocytoma. Int J Genomics 2018;2018:6582014. [Crossref] [PubMed]
- Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013;23:739-52. [Crossref] [PubMed]
- Ladroue C, Carcenac R, Leporrier M, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med 2008;359:2685-92. [Crossref] [PubMed]
- Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005;366:665-75. [Crossref] [PubMed]
- Comino-Méndez I, Tejera AM, Curras-Freixes M, et al. ATRX driver mutation in a composite malignant pheochromocytoma. Cancer Genet 2016;209:272-7. [Crossref] [PubMed]
- Sanchez M, Galy B, Muckenthaler MU, et al. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol 2007;14:420-6. [Crossref] [PubMed]
- Wilkinson N, Pantopoulos K. IRP1 regulates erythropoiesis and systemic iron homeostasis by controlling HIF2alpha mRNA translation. Blood 2013;122:1658-68. [Crossref] [PubMed]
- Monné M, Palmieri F. Antiporters of the mitochondrial carrier family. Curr Top Membr 2014;73:289-320. [Crossref] [PubMed]
- Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am 2011;40:295-311. vii. [Crossref] [PubMed]
- Baxter MA, Hunter P, Thompson GR, et al. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf) 1992;37:304-6. [Crossref] [PubMed]
- Van Der Horst-Schrivers AN, Osinga TE, Kema IP, et al. Dopamine excess in patients with head and neck paragangliomas. Anticancer Res 2010;30:5153-8. [PubMed]
- Eisenhofer G, Huynh TT, Hiroi M, et al. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Rev Endocr Metab Disord 2001;2:297-311. [Crossref] [PubMed]
- Hickman PE, Leong M, Chang J, et al. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma. Pathology 2009;41:173-7. [Crossref] [PubMed]
- Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 2002;287:1427-34. [Crossref] [PubMed]
- Pamporaki C, Darr R, Bursztyn M, et al. Plasma-free vs deconjugated metanephrines for diagnosis of phaeochromocytoma. Clin Endocrinol (Oxf) 2013;79:476-83. [Crossref] [PubMed]
- Weismann D, Peitzsch M, Raida A, et al. Measurements of plasma metanephrines by immunoassay vs liquid chromatography with tandem mass spectrometry for diagnosis of pheochromocytoma. Eur J Endocrinol 2015;172:251-60. [Crossref] [PubMed]
- Rao D, Peitzsch M, Prejbisz A, et al. Plasma methoxytyramine: clinical utility with metanephrines for diagnosis of pheochromocytoma and paraganglioma. Eur J Endocrinol 2017;177:103-13. [Crossref] [PubMed]
- Eisenhofer G, Prejbisz A, Peitzsch M, et al. Biochemical Diagnosis of Chromaffin Cell Tumors in Patients at High and Low Risk of Disease: Plasma versus Urinary Free or Deconjugated O-Methylated Catecholamine Metabolites. Clin Chem 2018;64:1646-56. [Crossref] [PubMed]
- Därr R, Pamporaki C, Peitzsch M, et al. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: importance of supine sampling under fasting conditions. Clin Endocrinol (Oxf) 2014;80:478-86. [Crossref] [PubMed]
- Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915-42. [Crossref] [PubMed]
- Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem 2014;60:1486-99. [Crossref] [PubMed]
- Peaston RT, Graham KS, Chambers E, et al. Performance of plasma free metanephrines measured by liquid chromatography-tandem mass spectrometry in the diagnosis of pheochromocytoma. Clin Chim Acta 2010;411:546-52. [Crossref] [PubMed]
- Pillai D, Callen S. Pilot quality assurance programme for plasma metanephrines. Ann Clin Biochem 2010;47:137-42. [Crossref] [PubMed]
- Hannah-Shmouni F, Pacak K, Stratakis CA. Metanephrines for Evaluating Palpitations and Flushing. JAMA 2017;318:385-6. [Crossref] [PubMed]
- Eisenhofer G, Goldstein DS, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab 2003;88:2656-66. [Crossref] [PubMed]
- Davidson FD. Paracetamol-associated interference in an HPLC-ECD assay for urinary free metadrenalines and catecholamines. Ann Clin Biochem 2004;41:316-20. [Crossref] [PubMed]
- Bouhanick B, Fauvel J, Pont F. Biochemical misdiagnosis of pheochromocytoma in patients treated with sulfasalazine. JAMA 2010;304:1898-901. [Crossref] [PubMed]
- Osinga TE, Kema IP, Kerstens MN, et al. No influence of antihypertensive agents on plasma free metanephrines. Clin Biochem 2016;49:1368-71. [Crossref] [PubMed]
- Grebe SK, Singh RJ. LC-MS/MS in the Clinical Laboratory - Where to From Here? Clin Biochem Rev 2011;32:5-31. [PubMed]
- Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab 2007;92:4069-79. [Crossref] [PubMed]
- Isaacs M, Lee P. Preoperative alpha-blockade in phaeochromocytoma and paraganglioma: is it always necessary? Clin Endocrinol (Oxf) 2017;86:309-14. [Crossref] [PubMed]
- Conzo G, Musella M, Corcione F, et al. Role of preoperative adrenergic blockade with doxazosin on hemodynamic control during the surgical treatment of pheochromocytoma: a retrospective study of 48 cases. Am Surg 2013;79:1196-202. [PubMed]
- Groeben H, Nottebaum BJ, Alesina PF, et al. Perioperative alpha-receptor blockade in phaeochromocytoma surgery: an observational case series. Br J Anaesth 2017;118:182-9. [Crossref] [PubMed]
- Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and Paraganglioma. N Engl J Med 2019;381:552-65. [Crossref] [PubMed]
- Wolf KI, Santos JRU, Pacak K. Why Take the Risk? We Only Live Once: The Dangers Associated with Neglecting a Pre-Operative Alpha Adrenoceptor Blockade in Pheochromocytoma Patients. Endocr Pract 2019;25:106-8. [Crossref] [PubMed]
- Pai R, Manipadam MT, Singh P, et al. Usefulness of Succinate dehydrogenase B (SDHB) immunohistochemistry in guiding mutational screening among patients with pheochromocytoma-paraganglioma syndromes. APMIS 2014;122:1130-5. [PubMed]
- Papathomas TG, Oudijk L, Persu A, et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol 2015;28:807-21. [Crossref] [PubMed]
- Menara M, Oudijk L, Badoual C, et al. SDHD immunohistochemistry: a new tool to validate SDHx mutations in pheochromocytoma/paraganglioma. J Clin Endocrinol Metab 2015;100:E287-91. [Crossref] [PubMed]
- Udager AM, Magers MJ, Goerke DM, et al. The utility of SDHB and FH immunohistochemistry in patients evaluated for hereditary paraganglioma-pheochromocytoma syndromes. Hum Pathol 2018;71:47-54. [Crossref] [PubMed]
- Farhat NA, Powers JF, Shepard-Barry A, et al. A Previously Unrecognized Monocytic Component of Pheochromocytoma and Paraganglioma. Endocr Pathol 2019;30:90-5. [Crossref] [PubMed]
- Favier J, Meatchi T, Robidel E, et al. Carbonic anhydrase 9 immunohistochemistry as a tool to predict or validate germline and somatic VHL mutations in pheochromocytoma and paraganglioma-a retrospective and prospective study. Mod Pathol 2020;33:57-64. [Crossref] [PubMed]
- van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol 2009;10:764-71. [Crossref] [PubMed]
- Santi R, Rapizzi E, Canu L, et al. Potential Pitfalls of SDH Immunohistochemical Detection in Paragangliomas and Phaeochromocytomas Harbouring Germline SDHx Gene Mutation. Anticancer Res 2017;37:805-12. [Crossref] [PubMed]
- Gill AJ. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018;72:106-16. [Crossref] [PubMed]
- Al-Ahmadie HA, Alden D, Qin LX, et al. Carbonic anhydrase IX expression in clear cell renal cell carcinoma: an immunohistochemical study comparing 2 antibodies. Am J Surg Pathol 2008;32:377-82. [Crossref] [PubMed]
- Pinato DJ, Ramachandran R, Toussi ST, et al. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. Br J Cancer 2013;108:429-37. [Crossref] [PubMed]
- Jochmanova I, Pacak K. Pheochromocytoma: The First Metabolic Endocrine Cancer. Clin Cancer Res 2016;22:5001-11. [Crossref] [PubMed]
- Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol 2015;11:101-11. [Crossref] [PubMed]
- Griffin JL. Metabonomics: NMR spectroscopy and pattern recognition analysis of body fluids and tissues for characterisation of xenobiotic toxicity and disease diagnosis. Curr Opin Chem Biol 2003;7:648-54. [Crossref] [PubMed]
- Lendvai N, Pawlosky R, Bullova P, et al. Succinate-to-fumarate ratio as a new metabolic marker to detect the presence of SDHB/D-related paraganglioma: initial experimental and ex vivo findings. Endocrinology 2014;155:27-32. [Crossref] [PubMed]
- Richter S, Gieldon L, Pang Y, et al. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genet Med 2019;21:705-17. [Crossref] [PubMed]
- Richter S, Peitzsch M, Rapizzi E, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab 2014;99:3903-11. [Crossref] [PubMed]
- Imperiale A, Moussallieh FM, Roche P, et al. Metabolome profiling by HRMAS NMR spectroscopy of pheochromocytomas and paragangliomas detects SDH deficiency: clinical and pathophysiological implications. Neoplasia 2015;17:55-65. [Crossref] [PubMed]
- Kim E, Wright MJ, Sioson L, et al. Utility of the succinate: Fumarate ratio for assessing SDH dysfunction in different tumor types. Mol Genet Metab Rep 2016;10:45-9. [Crossref] [PubMed]
- Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 2005;14:2231-9. [Crossref] [PubMed]
- Alrezk R, Suarez A, Tena I, et al. Update of Pheochromocytoma Syndromes: Genetics, Biochemical Evaluation, and Imaging. Front Endocrinol (Lausanne) 2018;9:515. [Crossref] [PubMed]
- Mercado-Asis LB, Wolf KI, Jochmanova I, et al. Pheochromocytoma: A Genetic and Diagnostic Update. Endocr Pract 2018;24:78-90. [Crossref] [PubMed]
- Mei L, Khurana A, Al-Juhaishi T, et al. Prognostic Factors of Malignant Pheochromocytoma and Paraganglioma: A Combined SEER and TCGA Databases Review. Horm Metab Res 2019;51:451-7. [Crossref] [PubMed]
- Taïeb D, Pacak K. Current experts' views on precision nuclear medicine imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 2019;46:2223-4. [Crossref] [PubMed]
- Čtvrtlík F, Koranda P, Schovanek J, et al. Current diagnostic imaging of pheochromocytomas and implications for therapeutic strategy. Exp Ther Med 2018;15:3151-60. [PubMed]
- Taïeb D, Hicks RJ, Hindie E, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 2019;46:2112-37. [Crossref] [PubMed]
- Archier A, Varoquaux A, Garrigue P, et al. Prospective comparison of (68)Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. Eur J Nucl Med Mol Imaging 2016;43:1248-57. [Crossref] [PubMed]
- Janssen I, Blanchet EM, Adams K, et al. Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res 2015;21:3888-95. [Crossref] [PubMed]
- Janssen I, Chen CC, Taieb D, et al. 68Ga-DOTATATE PET/CT in the Localization of Head and Neck Paragangliomas Compared with Other Functional Imaging Modalities and CT/MRI. J Nucl Med 2016;57:186-91. [Crossref] [PubMed]
- Fani M, Nicolas GP, Wild D. Somatostatin Receptor Antagonists for Imaging and Therapy. J Nucl Med 2017;58:61S-6S. [Crossref] [PubMed]
- Itani M, Mhlanga J. Imaging of Pheochromocytoma and Paraganglioma. In: Mariani-Costantini R. editor. Paraganglioma: A Multidisciplinary Approach. Brisbane: Codon Publications, 2019.
- Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res 2005;11:2785-808. [Crossref] [PubMed]
- Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2009;94:4757-67. [Crossref] [PubMed]
- Taïeb D, Timmers HJ, Shulkin BL, et al. Renaissance of (18)F-FDG positron emission tomography in the imaging of pheochromocytoma/paraganglioma. J Clin Endocrinol Metab 2014;99:2337-9. [Crossref] [PubMed]
- Mak IYF, Hayes AR, Khoo B, et al. Peptide Receptor Radionuclide Therapy as a Novel Treatment for Metastatic and Invasive Phaeochromocytoma and Paraganglioma. Neuroendocrinology 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Choi YM, Sung TY, Kim WG, et al. Clinical course and prognostic factors in patients with malignant pheochromocytoma and paraganglioma: A single institution experience. J Surg Oncol 2015;112:815-21. [Crossref] [PubMed]
- Kohlenberg J, Welch B, Hamidi O, et al. Efficacy and Safety of Ablative Therapy in the Treatment of Patients with Metastatic Pheochromocytoma and Paraganglioma. Cancers (Basel) 2019. [Crossref] [PubMed]
- Kong G, Grozinsky-Glasberg S, Hofman MS, et al. Efficacy of Peptide Receptor Radionuclide Therapy for Functional Metastatic Paraganglioma and Pheochromocytoma. J Clin Endocrinol Metab 2017;102:3278-87. [Crossref] [PubMed]
- Yadav MP, Ballal S, Bal C. Concomitant (177)Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res 2019;9:13. [Crossref] [PubMed]
- Jimenez C. Treatment for Patients With Malignant Pheochromocytomas and Paragangliomas: A Perspective From the Hallmarks of Cancer. Front Endocrinol (Lausanne) 2018;9:277. [Crossref] [PubMed]
- Pryma DA, Chin BB, Noto RB, et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J Nucl Med 2019;60:623-30. [Crossref] [PubMed]
- Carrasquillo JA, Pandit-Taskar N, Chen CC. I-131 Metaiodobenzylguanidine Therapy of Pheochromocytoma and Paraganglioma. Semin Nucl Med 2016;46:203-14. [Crossref] [PubMed]
- Gonias S, Goldsby R, Matthay KK, et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol 2009;27:4162-8. [Crossref] [PubMed]
- Vyakaranam AR, Crona J, Norlen O, et al. Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with (177)Lu-DOTATATE. Cancers (Basel) 2019. [Crossref]
- Makis W, McCann K, McEwan AJ. The Challenges of Treating Paraganglioma Patients with (177)Lu-DOTATATE PRRT: Catecholamine Crises, Tumor Lysis Syndrome and the Need for Modification of Treatment Protocols. Nucl Med Mol Imaging 2015;49:223-30. [Crossref] [PubMed]
- Pang Y, Lu Y, Caisova V, et al. Targeting NAD(+)/PARP DNA Repair Pathway as a Novel Therapeutic Approach to SDHB-Mutated Cluster I Pheochromocytoma and Paraganglioma. Clin Cancer Res 2018;24:3423-32. [Crossref] [PubMed]
- Hadoux J, Favier J, Scoazec JY, et al. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer 2014;135:2711-20. [Crossref] [PubMed]
- Nölting S, Garcia E, Alusi G, et al. Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J Mol Endocrinol 2012;49:79-96. [Crossref] [PubMed]
- Lee M, Waser B, Reubi JC, et al. Secretin receptor promotes the proliferation of endocrine tumor cells via the PI3K/AKT pathway. Mol Endocrinol 2012;26:1394-405. [Crossref] [PubMed]
- Lee M, Minaskan N, Wiedemann T, et al. Targeting PI3K/mTOR signaling exerts potent antitumor activity in pheochromocytoma in vivo. Endocr Relat Cancer 2017;24:1-15. [Crossref] [PubMed]
- Ballinger JR. Theranostic radiopharmaceuticals: established agents in current use. Br J Radiol 2018;91:20170969. [Crossref] [PubMed]
- Makis W, McCann K, McEwan AJ, et al. Palliation of Extensive Metastatic Bone Disease With 223Ra-Dichloride alpha-Particle Therapy in a Patient With Malignant Hereditary Paraganglioma-Pheochromocytoma Syndrome With SDHB Mutation. Clin Nucl Med 2016;41:144-7. [Crossref] [PubMed]
- Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 2016;17:1306-16. [Crossref] [PubMed]
- Aita Y, Ishii KA, Saito Y, et al. Sunitinib inhibits catecholamine synthesis and secretion in pheochromocytoma tumor cells by blocking VEGF receptor 2 via PLC-gamma-related pathways. Am J Physiol Endocrinol Metab 2012;303:E1006-14. [Crossref] [PubMed]
- Ayala-Ramirez M, Chougnet CN, Habra MA, et al. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab 2012;97:4040-50. [Crossref] [PubMed]
- Caisova V, Li L, Gupta G, et al. The Significant Reduction or Complete Eradication of Subcutaneous and Metastatic Lesions in a Pheochromocytoma Mouse Model after Immunotherapy Using Mannan-BAM, TLR Ligands, and Anti-CD40. Cancers (Basel) 2019. [Crossref] [PubMed]


