The impact of overweight and obesity on breast cancer: data from Switzerland, so far a country little affected by the current global obesity epidemic
Introduction
Breast cancer (BC) is the most common cancer in women. In Switzerland, 20% of the deaths caused by cancer are due to BC (1). In most epidemiological studies obesity is associated with an increased risk of postmenopausal BC, while an inverse relationship is observed in premenopausal women (2).
Overweight and obesity are considered at the present to be the most important nutritional health risk in Western countries. Excessive body fatness is not only a risk factor for cardiovascular diseases and diabetes, but also for different types of cancers. In the last decades overweight and obesity have become a worldwide epidemic. The International Association for the Study of Obesity (IASO) estimates that 300 million people around the world are obese and expects that obesity levels will continue to rise in the early 21st century and shortly become a global epidemic (3). The Food and Agricultural Organization of the United Nations points out that this problem does not only affect western countries as a considerable problem but, increasingly, developing countries as well (“the developing world’s new burden: obesity”) (4).
The impact of obesity on BC is complex and not yet completely understood (5) (for questions as to whether obesity itself is conductive to an environment of carcinogenesis, please see Box 1). There is not only an increase in postmenopausal BC in obese women, in addition, being obese has been shown to influence BC prognosis adversely (2,5-7,9-13) It has been suggested that a more advanced disease stage (12), more aggressive tumor characteristics at diagnosis (6) and suboptimal local and systemic treatment (14) of obese women in relation to and in comparison with patients of normal weight could explain the poorer prognosis.
In this review, we present the data and results from our project “The Impact of Overweight and Obesity on Breast Cancer: Data from Switzerland” which was initiated in 2009 at the University Hospital Basel and the Institute of Social and Preventive Medicine (Division of Cancer Epidemiology and Prevention) of the University of Zürich.
The entire project comprised three major issues:
- The role of overweight and obesity in the etiology of BC (15);
- The relationship between overweight/obesity to diagnosis and the presenting characteristics of the tumor (16);
- The impact of BMI on patient compliance and persistence towards adjuvant BC therapy (17).
Our Swiss data is interesting because the general Swiss female population is distinctive in two areas compared to that of most other industrialized countries: (I) Switzerland has comparatively low rates of overweight (22-23%) and obesity (7-8%) in adult women and (II) has rather stable rates of overweight and obesity (18-20).
Data regarding the prevalence and development of overweight and obesity in Switzerland have been recorded since 1992 every 5 years by the Schweizerisches Bundesamt für Statistik (Swiss Federal Office of Statistics) within the Schweizerische Gesundheitsbefragungen (Swiss Health Surveys, SHS). In 2007, the fourth data collection was done. The existing four surveys, from 1992, 1997, 2002 and in 2007, were each carried out in representative, randomly selected samples comprising 20,000 to 30,000 private households with a telephone connection to represent the Swiss permanent population, i.e., male and female Swiss citizens and foreigners with a legal work permit aged 15 years and older (21).
In the survey from 2007, it was found that 21.7% of adult females were overweight [ow, body mass index (BMI) ≥25-29.9] and an additional 8.1% were obese (ob, BMI ≥30); in total, 29.8% of women in Switzerland showed a BMI ≥25 (20). Even if one considers that these Swiss results are self-reported data and that these, taken at face value, tend to underestimate obesity prevalence when compared to actual measured data (22), Switzerland is, in comparison with international data, certainly in the lower range. The IASO has compiled the national data for the 27 countries of the European Union. On average, 47.5% of adult females showed a BMI ≥25 (ow: 29.5%; ob: 18.1%) (23). The USA clearly exceeds the European values with 61.8% of females with a BMI ≥25 (ow: 28.6%; ob: 33.2%) (24).
Notable in the Swiss results is not only the comparatively low prevalence of overweight and obesity, but also that the data have remained relatively stable over the last ten years (1997: ow: 21.8%; ob: 7.2%. 2002: ow: 22.7%; 7.8%. 2007: see above) (18-20). Likewise, in a cross-sectional repeated survey of adults aged 35-74 years in Geneva, obesity prevalence did also not increase significantly in women between 1993 and 2004 (25). In contrast to this data, in nearly all other countries in the last 10-15 years, a considerable rise in obesity prevalence has been observed (“global epidemic”) (3,26,27).
The main BC data source of this project was the Basel Breast Cancer Database (BBCD). This web-based data documentation system records all newly diagnosed primary invasive BC cases treated at the University Women’s Hospital Basel, Switzerland since 1990. Data of in total 1,459 patients up to 2009 were used for this project. The cases and their individual features and variables are summarized and recorded in 18 different forms. All together, a maximum of 349 variables can be coded per case. A standard case, i.e., a case without distant metastases in the further course of disease, includes approximately 150 different variables: sociodemographic data, medical history, family history, clinical presentation at initial diagnosis, pathological characteristics, tumor staging, data on clinical management with reference to surgery, radiation and adjuvant and palliative treatment and follow-up status. The data are collected continuously. This database mirrors the development of BC diagnosis and treatment over a period of 20 years (e.g., increased diagnosis of non-palpable lesions by radiologic examinations, more breast-conserving surgery, trends in adjuvant systemic treatment, etc.). It is a particular strength of the BBCD project, that with a lost to follow-up rate of <3%, we have a complete documentation in nearly all cases.
From the 1,459 patients included in the BBCD, we had information on BMI in 97% of the cases (n=1,415). The clinicopathologic data of these 1,415 patients are listed in Table 1. The BMI was calculated using the following standard formula: body weight (kg)/height (m2). We used only directly measured BMI data taken at the time of initial BC diagnosis. Based on these data, BMI was calculated and categorized according to the WHO criteria as follows (31):
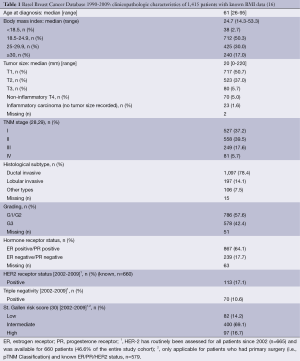
Full table
Data collection methods and the study design of this project were approved by the Ethical Review. The project was supported by the Krebsliga beider Basel; the sponsor had no influence whatsoever on the reported data.
Part I: the role of overweight and obesity in the etiology of postmenopausal breast cancer (15)
In 2007, the working group World Cancer Research Fund/American Institute for Cancer Research reviewed the literature extensively, and after analyzing approximately 200 studies, developed the following summary: while there is abundant and consistent epidemiological evidence and a clear dose response that greater body fatness is a risk factor for postmenopausal BC, it probably protects against premenopausal BC (2). For postmenopausal BC, a meta-analysis was possible on 17 cohort studies, giving a summary effect estimate of 1.03 (95% CI: 1.01-1.04 per 2 kg/m2). The observed high heterogeneity of the 17 cohort studies may be explained in part by not adjusting for hormone replacement therapy (HRT). There is some evidence that body fatness increases the risk only in women not taking HRT. Because exogenous sources of estrogen, such as HRT, artificially elevate a woman’s circulating levels, they may mask any effect of increased endogenous estrogen from body fat (2).
Even though there is a substantial amount of consistent epidemiological evidence, the potential mechanisms through which body fatness might prevent premenopausal BC remain speculative. Furthermore, because BC diagnosed post menopause is much more common, a decreased risk of premenopausal BC would be outweighed by an increased risk of postmenopausal BC (2).
With regard to the question of whether body fatness poses a risk factor for the development of postmenopausal BC, there was no published data from Switzerland and in the first part of our project, we addressed this issue. Although we had nearly complete information regarding the menopausal status of the patients in the BBCD, we do not have this information in the data from the SHS. Since the mean age of menopause in the BBCD was 50.06 years, we used an age-dependent inclusion criterion and analyzed women from 51-80 years of age. We were aware of the fact that using this method would lead to exclusion of certain groups (>51 years, premenopausal; <51 years, postmenopausal). However, with respect to the entire study group, the number of such patients is likely to be so small that it should not have a significant effect on the reported results.
Of all patients in the BBCD, 985 patients (65.7%) met our inclusion criteria; of these, information on BMI was available for 958 women; the mean age of this study subgroup was 65.0 years. From the 35,090 women who were interviewed in the SHS and provided information on BMI, 14,476 women (37.2%) met our age-dependent inclusion criteria; the mean age of this subgroup was 63.7 years.
In this study, we compared objective BMI data (BBCD: measured) with subjective ones (SHS: self-reported). Since individuals included in population studies tend to underreport their weight and overestimate their height, obesity prevalence based on this data is often inaccurate (22,32). Since over- and under-reporting appears to be quite systematic, correction factors using separate adjustment factors resulted in an increase in the accuracy of self-reported estimates (22,33,34). In this study, we used an equation developed by Hayes et al. (34). This calculation model was tested and approved for the Swiss population (35).
In the BBCD, data on body weight and height have been collected continuously since 1990. In the SHS, on the contrary, these data were only collected in the four years that the surveys were performed, namely 1992, 1997, 2002 and 2007. In order to evaluate BMI distribution time trends over the last 20 years, we defined the four following subgroups:
- BBCD 1990-1994 vs. SHS 1992;
- BBCD 1995-1999 vs. SHS 1997;
- BBCD 2000-2004 vs. SHS 2002;
- BBCD 2005-2009 vs. SHS 2007.
The BMI distributions of the BBCD and the SHS study cohorts are shown in Table 2. Of the BBCD group, 309 women (32.3%) were overweight and 188 women (19.6%) were obese at the time of the initial BC diagnosis. This data is comparable with a group of 337 BC patients >50 years who were registered in the Canton of Geneva between 2003 to 2005 and of which 28% were overweight and 21% were obese (36).
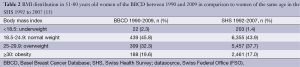
Full table
In comparison, of women of the same age in the overall group of the SHS 1992 to 2007, 37.7% were overweight and 17.0% were obese. During the above mentioned four time periods considered in the BBCD, the prevalence of overweight were 35.5%, 36.3%, 31.3% and 27.8%, respectively; the prevalence of obesity were 15.0%, 23.8%, 18.9%, and 21.2%, respectively (Table 3). For 51-80-year-old women of the four SHS of 1992, 1997, 2002 and 2007, the corresponding prevalence for overweight and obesity, corrected for self-report, were 38.6%, 37.8%, 38.5%, 36.2% and 12.9%, 19.5%, 17.4% and 17.7%, respectively (Table 3).
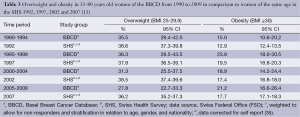
Full table
Statistical tests for the overall course of time over the four periods revealed a comparable pattern in all tested categories of the SHS and the BBCD, respectively. For the category “overweight”, the tests for the overall course of time were not statistically significant. For the category “obesity”, there was a transient increase over time in the SHS (overweight and obesity increased between 1992 and 1997. However, between 1997 and 2007, a statistically significant increase was no longer observed). The quadratic contrast was significant and negative, indicating a convex curvature, as can be seen in Figure 1. For the BBCD data, however, the test for the overall period of time was not statistically significant. Overall, there was no evidence of statistically significant differences between BBCD data and corrected and weighted SHS data in relation to overweight and obesity over the four time periods considered.

The existence of a consistent association between obesity and BC risk in countries with high obesity prevalence rates has been demonstrated time and again in the literature (2). This seems to indicate that there must be a higher prevalence of overweight and obesity in BC patients than in women of the same age in the general population. In the present analysis, however, no such difference was observed for our Swiss study group. This data does not correspond to that of most other industrialized countries, which have been affected by a considerable rise in obesity prevalence (37-42). A possible explanation for this observation may be a curvilinear dose-response relationship between BMI and postmenopausal BC risk (2), so that an increased risk may only be observed in populations with a high prevalence of obese and very obese women.
Our data shows that findings from countries with high overweight/obesity prevalence may not unconditionally be applied to populations with comparably rather low overweight/obesity prevalence such as Switzerland. Compared to the USA, for example [approximately 50% of the cohort studies compiled in the WCRF/AICR review stem from the USA (2)], the obesity rates in Switzerland are actually half as high (43). It might be hypothesized that the association between obesity and BC risk cannot be explained by the factor BMI alone but also by a considerably different lifestyle (including numerous other insufficiently controllable other factors that might also facilitate carcinogenesis) that possibly also contribute to a condition where there are significantly more women with excessive body fatness.
A further explanation is that there might be a relationship between the increased risk of postmenopausal BC and central obesity. A Dutch study demonstrated that there was a correlation between central fat tissue and postmenopausal BC whereas there was no correlation between BC risk and BMI alone (44). This data is in line with study results from Italy which showed that there were more women with a metabolic syndrome in postmenopausal BC and that the level of central fat tissue increased the risk for BC significantly, again with no correlation for BMI alone (45).
Part II: the impact of BMI on prognostically relevant breast cancer tumor characteristics (16)
For this second part of our project, we used the data of all patients of the BBCD with known BMI data (n=1,415 patients). The predictor variable of this study was directly measured BMI data taken at the time of initial BC diagnosis. In a multivariate analysis, the patient’s age at the time of diagnosis was also considered. We evaluated the impact of BMI and age on the following prognostically relevant factors: tumor size (tumor detected by self-palpation or by radiological examination) and stage (28,29), histological subtype, grading, hormonal receptor status, HER2 status, “triple-negative” status (hormonal receptor and HER2 status negative) and the risk score defined at the St. Gallen Expert Consensus Meeting in 2007 (30). Furthermore, we considered the tumor detection method and evaluated three different methods: self-detection, clinical breast examination and radiological breast examination including mammography and sonography.
As demonstrated in Table 4 and Figure 2, our data showed that BMI was significantly associated with tumor size (all patients: increase by 3 mm per 5 unit change in BMI: beta-coefficient =0.03, P<0.001); this applied not only to the cases where the tumor was found by self-detection (P<0.001), but also to lesions detected by radiological breast examinations (P=0.044). In addition, a higher BMI was positively correlated with advanced TNM stage, unfavorable grading and a higher St. Gallen risk score. No associations were observed between BMI and histological subtype, estrogen receptor (ER) status, HER2 status and triple negative BC. Higher age, on the other hand, increased the probability of lobular instead of ductal BC, less triple negative BC, ER positivity and more favorable grading. In the subgroup of postmenopausal women, the above mentioned findings were attenuated but did not change the direction of the associations (Table 4).
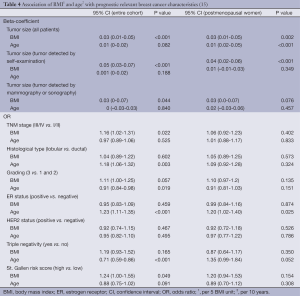
Full table
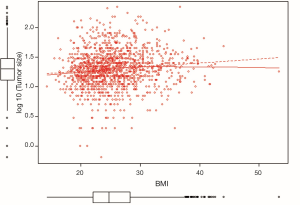
Positive correlation with BMI in our cohort: tumor size, TNM stage, grading, St. Gallen risk score
There is increasing evidence that BMI is inversely associated with BC prognosis (2,5-7,9-13). It has been suggested that larger tumor size, more advanced stage and grade of the tumor at diagnosis (12) could explain in part this bad outcome. The findings of several studies are in accordance with this hypothesis (11,46); however, others are not (47). Accordingly, Wasserman et al. (48) observed no association between BMI and disease stage at diagnosis in 301 postmenopausal women of the Women’s Healthy Eating and Living study. The same was true for women of any age in another study (49). Similarly, in the study by Chagpar et al., no association was observed between BMI and tumor size, lymph node status, or disease stage at diagnosis in mostly postmenopausal women with hormonally sensitive BC (47). In contrast, several other studies observed a positive association between BMI and disease stage, tumor size and lymph node status at diagnosis (10,46,50), as we did in our survey for tumor size and stage. In a study carried out in the Canton of Geneva, Switzerland (36), invasive carcinomas >1 cm were more frequently impalpable in obese women (22%) than in normal/underweight women (12%). It may be more difficult for clinicians and women to palpate a tumor in a large breast than in a small one. Thus, obese women with larger breasts may have larger tumors and more advanced tumor stages at diagnosis than lean women with small breasts. Accordingly, in a study of 2,863 patients diagnosed with BC in Wisconsin, elevated BMI was associated with a greater probability of non-localized tumors in self-detected cancers, but in women, whose tumors were found by screening mammography or by clinical breast examination, BMI was not related to disease stage (51). In our Swiss cohort, however, BMI was found to be a significant factor for being diagnosed with larger tumors, both for lesions found by self-detection, as well as for those detected by radiological breast examinations. This could mean that either both methods for tumor detection are impaired by large breasts, or larger tumors in larger breasts could be the consequence of obesity.
Non-adherence to BC screening is another possible explanation for our findings. The complex relationship between BMI and patient acceptance of the necessity of medical measures is well-examined with regard to mammography screening rates. Obese women are less likely to follow physician’s recommendations for breast and cervical cancer screening (52). On the basis of ten studies, Cohen et al. found among women in the U.S. that obesity is most likely a barrier to screening for BC (53). Some authors reported significantly less compliance to BC screening in obese women (52,54,55), whereas others did not confirm this finding in a large population-based analysis of more than 130,000 women (56). The reasons why a significant number of obese patients delay or refuse to participate in cancer screening programs are not yet fully understood. One of the most common reasons women give is the embarrassment of being weighed or having to undergo a physical examination with even more embarrassing aspects to endure (e.g., too small gowns, examination tables, instruments) (52,55,57). In our cohort, we could not comment on this explanation because up until now there exists only opportunistic screening in the Basel region.
No correlation between BMI and estrogen receptor status, HER2 status and triple-negative carcinoma in our cohort
Apart from the factors of presentation at a more advanced stage of disease at diagnosis and/or the difficulty in palpating the tumors, the observed worse outcome of obese BC patients may also be explained by the more unfavorable tumor characteristics in these patients. There are some data to support this hypothesis. Obesity is accompanied by the up-regulation of various cellular proliferation pathways (8). Adipokines and estrogens, produced in adipose tissue, may enhance tumor cell proliferation and metastasis (8,58,59), and may potentially result in more aggressive ER-positive cancers in post-menopausal women (60).
The evidence of a positive association between body size and ER-positive BC is quite consistent (61-63). In a cohort study including 155,723 women of the Women’s Health Initiative, Phipps et al. found an increased risk of triple-negative BC and ER-positive carcinomas for women in the highest versus lowest BMI quartile (64). Even though the ER-positive BC subtype has a rather good prognosis, obesity in postmenopausal women may result in more biologically aggressive ER positive tumors (60,65).
Obesity may affect BC risk by increasing circulating endogenous estrogen levels (66). Thus, the association between body weight and BC risk may be modified by the tumor’s ER and PR status. Accordingly, the summarized findings of nine cohort and 22 case-control studies comparing the highest versus the reference categories of relative body weight observed that the risk for ER+PR+ tumors was 20% lower among premenopausal and 82% higher among postmenopausal women (63). No associations were observed for ER-PR- or ER+PR- tumors. More recent studies confirmed these findings (5,64,67,68). The association between BMI and BC risk is thus dependent on the tumor’s ER/PR status and the woman’s menopausal status, and in some studies the risk was modified by HRT (68-70).
The HER2 status may be inversely associated with BMI independent of ER status (71), as shown in a study of postmenopausal women. Phipps et al. on the other hand, found no difference in the association between BMI and HER2 status (64). Additional studies with sufficient numbers of HER2-positive and HER2-negative tumors are needed to clarify the association between HER2 status and BMI.
Data examining obesity as a risk factor for triple negative BC are limited and inconsistent (72,73). Case-control studies that evaluated BMI in women irrespective of menopausal status and in postmenopausal women found no association with TNBC (73-76). The pooling of two case-control studies revealed a positive association between BMI and triple negative BC (OR, 2.7) in postmenopausal women not using hormone replacement therapy (75); no association was observed in HRT users. In a prospective study Phipps et al. observed for postmenopausal women in the highest versus the lowest BMI quartile a 1.35-fold non-significantly increased risk of triple-negative carcinomas without association with HRT use (64).
Part III: the impact of BMI on patient compliance and persistence towards adjuvant breast cancer therapy (17)
“Overweight patients have less willpower and are less concerned about their health than non-obese patients”. There exists a great deal of negative stereotypical thinking regarding overweight and obese individuals and even physicians and care personnel are not immune to these commonly held stereotypical attitudes. Medical literature reports a considerable amount of evidence of these attitudes in doctors and nurses towards obese patients and this can be interpreted as an attitude of diminished respect for obese patients (52,77,78). This attitude is clearly felt by the patients, influencing them in their behavior and decisions (77-79). In order to test the above mentioned stereotype, we analyzed the impact of BMI on patient compliance and persistence towards adjuvant BC therapy.
For this last part of our project, we used BBCD data concerning all patients with non-metastatic invasive BC, who received surgical therapy between 1997 and 2009. We restricted the analysis to women who were ≤75 years old at initial BC diagnosis. In total, 772 patients met these inclusion criteria. We had no BMI information for six of the 772 patients; thus, 766 patients comprised our study cohort (Table 5).

Full table
We deliberately analyzed the period since 1997. In doing that, we could make certain that our data reflects the current treatment situation as it covers a period in which most of the currently valid guidelines of treatment were already active (30):
- Surgery/radiation: from the beginning of our study period, the concept of breast conserving therapy (BCT) was implemented to the extent that approximately two thirds of the patients were treated by BCT. Postoperative radiation is an integral part of the BCT concept; thus, high BCT rates imply high rates of adjuvant radiation.
- Chemotherapy: the introduction of new agents (above all the taxanes), as well as considerable advances in supportive care (above all the introduction of anti-emetics such as the 5HT3 antagonists) led to a situation where highly effective therapy concepts could be better implemented.
- Immunotherapy: trastuzumab was established as a standard treatment in patients with HER2-positive carcinomas.
- Endocrine therapy: in the late 1990s, endocrine therapy has become the gold standard not only for patients with advanced stage disease, but also for almost all patients with HR positive carcinomas. Furthermore, the introduction of the third-generation aromatase inhibitors in the adjuvant setting meant a crucial development in BC management.
- The treatment recommendations for all patients were based on the decision of the interdisciplinary tumor board of the University Hospital Basel. Bearing in mind the above mentioned negative stereotypes regarding overweight/obese patients, it is crucial for therapy recommendations to be made by an unprejudiced board, thereby averting a subjective decision by an individual physician.
We had complete information regarding the type of surgery carried out and the receipt of adjuvant chemotherapy, and/or radiation. As of 1997, adjuvant endocrine therapy has been the standard recommendation for all HR-positive patients, with few exceptions; the reasons not to recommend endocrine therapy included (I) a low-risk constellation (pT1a/b N0, favorable grading) and/or (II) advanced age of the patient with considerable co-morbidity.
We had complete information regarding the entire time period of adjuvant therapy for 758 patients of our study cohort (99.0%); eight patients (1.0%) were lost to follow-up.
A positive strength of our study was that we provided careful and detailed clinical follow-up with clear differentiation of the situations that led to denial or discontinuation of therapy; particular attention was given to a precise recording of the reasons for modifications and discontinuations.
In this study, we defined “compliance” as the readiness of the patient to accept a proposed adjuvant treatment or drug regimen. When the patients started the treatment, we used the term “persistence” (defined as the length of time from initiation to discontinuation of treatment) for the further application of therapy or intake of the drug regimen. According to previous studies on patient compliance and persistence towards endocrine adjuvant BC therapy in postmenopausal patients (80,83-86), we interpreted non-persistence mostly as an intentional action of the patients. According to this principle, patients who had to stop therapy due to local or systemic BC recurrence were not defined as non-persistent. The same holds true for cases where a physician decided to stop the therapy for medical reasons other than BC (e.g., in palliative situation of malignant diseases, dependence on nursing care, severe dementia). Furthermore, in accordance with other publications, patients who died from inter-current illness, having received therapy or medication shortly before death, were not considered non-persistent. We place a great deal of emphasis on the above mentioned criteria because non-persistence as an intentional action may be preventable by more intensive care and improved counseling and we believe that a certain proportion of such patients may potentially be motivated to continue therapy. This option is clearly not available in a situation where the discontinuation of therapy was not a choice but was mandatory, e.g., due to the above mentioned situations.
There are few published studies that evaluated the impact of body weight on patient compliance and persistence towards BC therapy (5,87,88). Deglise et al. found no differences in the use of systemic adjuvant BC treatment between BMI categories of 460 patients (5). The authors defined “use of systemic treatment” if the respective treatment was prescribed as noted in the Geneva cancer registry; however, recommendation and prescription of a therapy does not necessarily mean an actual induction (compliance) and completion (persistence) of therapy. In a more recent study, Maskarinec et al. assessed the compliance with recommended treatment guidelines in 382 BC patients and found that the majority of obese patients did receive the recommended adjuvant treatment (88). The group of patients in which significantly less adjuvant treatment was given was the group of older patients. Buist and colleagues restricted their analysis as to whether the receipt of appropriate adjuvant therapy in BC patients differs by BMI to 897 patients ≥65 years and found that adequate adjuvant therapy was not associated with BMI (87).
Our data go even further. With respect to compliance, multivariate analyses calculated Odds ratios (ORs) >1 for increased BMI in all four therapy modalities, i.e., increased BMI had a positive influence on patient compliance (Table 6): the results were significant for radiotherapy (OR, 2.37; 95% CI, 1.45-3.88; P<0.001) and endocrine therapy (OR, 1.92; 95% CI, 1.21-3.04; P=0.002) and showed a trend in chemotherapy (OR, 1.42; 95% CI, 0.97-2.08; P=0.063). In the analysis of data relating to therapy persistence, increasing BMI had ORs <1 for chemotherapy, without reaching statistical significance. For endocrine therapy, increasing BMI was a significant predictor for persistence (OR, 1.35; 95% CI, 1.08-1.80; P=0.042) (Table 7). We did not analyze persistence rates of radiotherapy: once the radiation was started (n=576), only very few patients (n=2, 0.3%) were non-persistent towards therapy (Table 5).
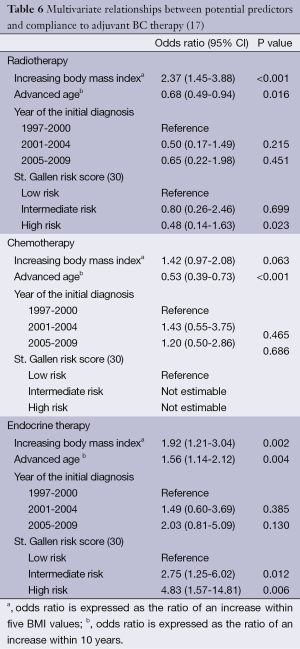
Full table
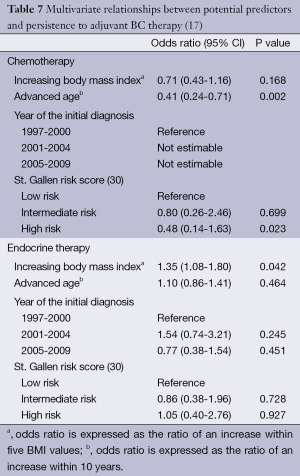
Full table
A possible reason for the high compliance (for radiotherapy, chemotherapy and endocrine therapy) and persistence (for endocrine therapy) in patients with increased BMI may be that this group’s somewhat strained relationship to screening and health education programs (6,52,54) as well as health care providers (77-79) does resolve itself once the hurdle of primary BC treatment is taken. Another factor could be that facing a new situation, which requires long-term medication such as adjuvant endocrine therapy, overweight/obese patients who often have to cope with other health issues due to co-morbidities requiring long-time medications (e.g., diabetes, hypertonia) are more reliable when it comes to taking an additional drug. Several studies reported a significant association between persistence with endocrine therapy and increasing numbers of other prescribed medications (89-91). It has been suggested that patients with a large medication burden develop routines to promote persistence and adherence (89,91).
As mentioned above, there is overwhelming evidence that overweight and obesity are associated with poor prognosis for both pre- and post-menopausal BC (2,5-7,9-13). The underlying mechanisms that can lead to decreased disease-specific and overall survival in these BC cancer patients are multiple and not yet entirely understood. In addition to the well-studied tumor-related characteristics of larger tumor size, more advanced stage of the disease and more aggressive tumor characteristics at diagnosis, there are host- and treatment-related factors contributing to the worse outcome in overweight/obese patients. Higher estrogen and testosterone hormone levels as well as concomitant hyperinsulinemia constitute a tumor-promoting environment and may therefore contribute to the patient’s poor survival (6,92). There are, however, contributing factors that are directly influenced either by the patient or the treating physician such as delayed or not-followed screening programs, reduced physical activity and weight gain (6,52,54) as well as the systematic under-treatment by empiric dose reductions of chemotherapy in overweight/obese BC patients (93,94).
The failure of the patient to comply and persist with adjuvant therapy, however, is not a contributing factor for the observed unfavorable prognosis in overweight/obese BC patients. In most therapy modes, patients with increasing BMI demonstrated a higher motivation and perseverance to the recommended treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bundesamt für Statistik (BFS), National Institute for Cancer Epidemiology and Registration (NICER), Schweizer Kinderkrebsregister (SKKR), Herausgeber. Krebs in der Schweiz. Stand der Entwicklung von 1983 bis 2007. Neuchatel: Bundesamt für Statistik, 2011:1-92.
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR, 2007:218-21.
- International Association of Obesity (IASO). The Global Epidemic. 2009. Available online: http://www.iotf.org/globalepidemic.asp
- Food and Agricultural Organisation of the United Nations. The developing world’s new burden: obesity. Available online: http://www.fao.org/focus/e/obesity/obes1.htm
- Deglise C, Bouchardy C, Burri M, et al. Impact of obesity on diagnosis and treatment of breast cancer. Breast Cancer Res Treat 2010;120:185-93. [PubMed]
- Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. Bjog 2006;113:1160-6. [PubMed]
- Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast 2004;13:85-92. [PubMed]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579-91. [PubMed]
- Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol 2002;20:1128-43. [PubMed]
- Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol 2008;26:4072-7. [PubMed]
- Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1686-91. [PubMed]
- Majed B, Moreau T, Senouci K, et al. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat 2008;111:329-42. [PubMed]
- Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134. [PubMed]
- Enger SM, Greif JM, Polikoff J, et al. Body weight correlates with mortality in early-stage breast cancer. Arch Surg 2004;139:954-8; discussion 958-60. [PubMed]
- Eichholzer M, Schmid SM, Bovey F, et al. Impact of overweight and obesity on postmenopausal breast cancer: analysis of 20-year data from Switzerland. Arch Gynecol Obstet 2012;285:797-803. [PubMed]
- Eichholzer M, Huang DJ, Modlasiak A, et al. Impact of body mass index on prognostically relevant breast cancer tumor characteristics. Breast Care (Basel) 2013;8:192-8. [PubMed]
- Schmid SM, Eichholzer M, Bovey F, et al. Impact of body mass index on compliance and persistence to adjuvant breast cancer therapy. Breast 2012;21:487-92. [PubMed]
- Eichholzer M, Bernasconi F, Jordan P, et al. Nutrition in Switzerland 2002-results of the Swiss Health Survey. Praxis (Bern 1994) 2005;94:1713-21.
- Eichholzer M, Bisig B, Gutzwiller F, et al. Aktuelle Ernährungsprobleme in der Schweiz. Resultate der Schweizerischen Gesundheitsbefragung 1997. Mitt Lebensm Unters Hyg 2000;91:251-73.
- Eichholzer M, Bovey F, Jordan P, et al. Data on overweight and nutrition in the 2007 Swiss Health Survey. Praxis (Bern 1994) 2010;99:17-25.
- Die Bundesbehörden der Schweizerischen Eidgenossenschaft. Erhebungen, Quellen-Schweizerische Gesundheitsbefragung (SGB). Available online: http://www.bfs.admin.ch/bfs/portal/de/index/infothek/erhebungen_quellen/blank/blank/ess/01.html
- Visscher TL, Viet AL, Kroesbergen IH, et al. Underreporting of BMI in adults and its effect on obesity prevalence estimations in the period 1998 to 2001. Obesity (Silver Spring) 2006;14:2054-63. [PubMed]
- International Association of Obesity (IASO). Overweight and Obesity in the EU27. Available online: http://www.iotf.org/database/documents/v2PDFforwebsiteEU27.pdf
- International Association of Obesity (IASO). Global Prevalence of Adult Obesity. Available online: http://www.iaso.org/site_media/uploads/Global_Prevalence_of_Adult_Obesity_January_2011.pdf
- Wolff H, Delhumeau C, Beer-Borst S, et al. Converging prevalences of obesity across educational groups in Switzerland. Obesity (Silver Spring) 2006;14:2080-8. [PubMed]
- International Association of Obesity (IASO). Increasing Obesity Rates in Europe 1985-2008. Available online: http://www.iaso.org/site_media/uploads/TrendsEuropeanadultsthroughtimeMay09.pdf
- World Health Organization (WHO). Obesity: preventing and managing the global epidemic. WHO-Technical Report Series no. 894. Geneva: World Health Organisation, 2000.
- Edge S, Byrd D, Compton C, et al. eds. AJCC Cancer Staging Manual. New York: Springer, 2009.
- Sobin L, Gospodarowicz M, Wittekind C. eds. UICC: TNM classification of malignant tumors. Oxford: Wiley-Blackwell, 2009.
- Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007;18:1133-44. [PubMed]
- World Health Organization (WHO). Diet, nutrition and the prevention of chronic diseases. WHO-Technical Report Series no. 916. Geneva: World Health Organization, 2002.
- Connor Gorber S, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8:307-26. [PubMed]
- Connor Gorber S, Shields M, Tremblay MS, et al. The feasibility of establishing correction factors to adjust self-reported estimates of obesity. Health Rep 2008;19:71-82. [PubMed]
- Hayes AJ, Kortt MA, Clarke PM, et al. Estimating equations to correct self-reported height and weight: implications for prevalence of overweight and obesity in Australia. Aust N Z J Public Health 2008;32:542-5. [PubMed]
- Faeh D, Braun J, Bopp M. Underestimation of obesity prevalence in Switzerland: comparison of two methods for correction of self-report. Swiss Med Wkly 2009;139:752-6. [PubMed]
- Deglise C, Bouchardy C, Burri M, et al. Impact of obesity on diagnosis and treatment of breast cancer. Breast Cancer Res Treat 2010;120:185-93. [PubMed]
- Charafeddine R, Van Oyen H, Demarest S. Trends in social inequalities in obesity: Belgium, 1997 to 2004. Prev Med 2009;48:54-8. [PubMed]
- Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obes Rev 2005;6:11-2. [PubMed]
- Czernichow S, Vergnaud AC, Maillard-Teyssier L, et al. Trends in the prevalence of obesity in employed adults in central-western France: a population-based study, 1995-2005. Prev Med 2009;48:262-6. [PubMed]
- Neovius M, Janson A, Rossner S. Prevalence of obesity in Sweden. Obes Rev 2006;7:1-3. [PubMed]
- Schokker DF, Visscher TL, Nooyens AC, et al. Prevalence of overweight and obesity in the Netherlands. Obes Rev 2007;8:101-8. [PubMed]
- Andreyeva T, Michaud PC, van Soest A. Obesity and health in Europeans aged 50 years and older. Public Health 2007;121:497-509. [PubMed]
- Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2012;307:491-7. [PubMed]
- Kaaks R, Van Noord PA, Den Tonkelaar I, et al. Breast-cancer incidence in relation to height, weight and body-fat distribution in the Dutch “DOM” cohort. Int J Cancer 1998;76:647-51. [PubMed]
- Capasso I, Esposito E, Pentimalli F, et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther 2010;10:1240-3. [PubMed]
- Moorman PG, Jones BA, Millikan RC, et al. Race, anthropometric factors, and stage at diagnosis of breast cancer. Am J Epidemiol 2001;153:284-91. [PubMed]
- Chagpar AB, McMasters KM, Saul J, et al. Body mass index influences palpability but not stage of breast cancer at diagnosis. Am Surg 2007;73:555-60; discussion 560. [PubMed]
- Wasserman L, Flatt SW, Natarajan L, et al. Correlates of obesity in postmenopausal women with breast cancer: comparison of genetic, demographic, disease-related, life history and dietary factors. Int J Obes Relat Metab Disord 2004;28:49-56. [PubMed]
- Kim YA, Lee CW. Effects of obesity on breast cancer stage at diagnosis in Korean women. Eur J Cancer Prev 2004;13:13-7. [PubMed]
- Cui Y, Whiteman MK, Flaws JA, et al. Body mass and stage of breast cancer at diagnosis. Int J Cancer 2002;98:279-83. [PubMed]
- Reeves MJ, Newcomb PA, Remington PL, et al. Body mass and breast cancer. Relationship between method of detection and stage of disease. Cancer 1996;77:301-7. [PubMed]
- Ferrante JM, Chen PH, Crabtree BF, et al. Cancer screening in women: body mass index and adherence to physician recommendations. Am J Prev Med 2007;32:525-31. [PubMed]
- Cohen SS, Palmieri RT, Nyante SJ, et al. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer 2008;112:1892-904. [PubMed]
- Wee CC, McCarthy EP, Davis RB, et al. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med 2000;132:697-704. [PubMed]
- Fontaine KR, Heo M, Allison DB. Body weight and cancer screening among women. J Womens Health Gend Based Med 2001;10:463-70. [PubMed]
- Berz D, Sikov W, Colvin G, et al. ‘Weighing in’ on screening mammography. Breast Cancer Res Treat 2009;114:569-74. [PubMed]
- Destounis S, Newell M, Pinsky R. Breast imaging and intervention in the overweight and obese patient. AJR Am J Roentgenol 2011;196:296-302. [PubMed]
- Daling JR, Malone KE, Doody DR, et al. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 2001;92:720-9. [PubMed]
- Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol 2006;169:1505-22. [PubMed]
- Tchernof A, Despres JP. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res 2000;32:526-36. [PubMed]
- Enger SM, Ross RK, Paganini-Hill A, et al. Body size, physical activity, and breast cancer hormone receptor status: results from two case-control studies. Cancer Epidemiol Biomarkers Prev 2000;9:681-7. [PubMed]
- Colditz GA, Rosner BA, Chen WY, et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 2004;96:218-28. [PubMed]
- Suzuki R, Orsini N, Saji S, et al. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer 2009;124:698-712. [PubMed]
- Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev 2011;20:454-63. [PubMed]
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627-35. [PubMed]
- Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 2004;5:153-65. [PubMed]
- Setiawan VW, Monroe KR, Wilkens LR, et al. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol 2009;169:1251-9. [PubMed]
- Canchola AJ, Anton-Culver H, Bernstein L, et al. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control 2012. [Epub ahead of print]. [PubMed]
- Suzuki R, Rylander-Rudqvist T, Ye W, et al. Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer 2006;119:1683-9. [PubMed]
- Ritte R, Lukanova A, Berrino F, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res 2012;14:R76. [PubMed]
- Van Mieghem T, Leunen K, Pochet N, et al. Body mass index and HER-2 overexpression in breast cancer patients over 50 years of age. Breast Cancer Res Treat 2007;106:127-33. [PubMed]
- Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer 2012;2012:809291.
- Gierach GL, Burke A, Anderson WF. Epidemiology of triple negative breast cancers. Breast Dis 2010;32:5-24. [PubMed]
- Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008;109:123-39. [PubMed]
- Phipps AI, Malone KE, Porter PL, et al. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2008;17:2078-86. [PubMed]
- Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev 2007;16:439-43. [PubMed]
- Price JH, Desmond SM, Krol RA, et al. Family practice physicians’ beliefs, attitudes, and practices regarding obesity. Am J Prev Med 1987;3:339-45. [PubMed]
- Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res 2001;9:788-805. [PubMed]
- Huizinga MM, Cooper LA, Bleich SN, et al. Physician respect for patients with obesity. J Gen Intern Med 2009;24:1236-9. [PubMed]
- Güth U, Huang DJ, Schotzau A, et al. Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. Br J Cancer 2008;99:428-33. [PubMed]
- Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008;26:556-62. [PubMed]
- Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 2003;21:602-6. [PubMed]
- Güth U, Myrick ME, Kandler C, et al. The use of adjuvant endocrine breast cancer therapy in the oldest old. Breast 2013;22:863-8. [PubMed]
- Güth U, Myrick ME, Kilic N, et al. Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Res Treat 2012;131:491-9. [PubMed]
- Güth U, Myrick ME, Schötzau A, et al. Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: how often and how often does it work? Breast Cancer Res Treat 2011;129:799-807. [PubMed]
- Myrick ME, Schmid SM, Kilic N, et al. Eligibility, compliance and persistence of extended adjuvant endocrine therapy for breast cancer. Acta Oncol 2012;51:247-53. [PubMed]
- Buist DS, Ichikawa L, Prout MN, et al. Receipt of appropriate primary breast cancer therapy and adjuvant therapy are not associated with obesity in older women with access to health care. J Clin Oncol 2007;25:3428-36. [PubMed]
- Maskarinec G, Pagano I, Lurie G, et al. Factors affecting survival among women with breast cancer in Hawaii. J Womens Health (Larchmt) 2011;20:231-7. [PubMed]
- Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 2006;99:215-20. [PubMed]
- Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol 2004;22:3309-15. [PubMed]
- Barron TI, Connolly R, Bennett K, et al. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer 2007;109:832-9. [PubMed]
- Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev 2006;7:333-40. [PubMed]
- Madarnas Y, Sawka CA, Franssen E, et al. Are medical oncologists biased in their treatment of the large woman with breast cancer? Breast Cancer Res Treat 2001;66:123-33. [PubMed]
- Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 2005;165:1267-73. [PubMed]



