
Hereditary causes of primary aldosteronism and other disorders of apparent excess mineralocorticoid activity
Introduction
Essential hypertension accounts for the vast majority of cases of hypertension in adults, while secondary causes of hypertension have a prevalence of 10–15% (1,2). Prevalence of secondary hypertension increases with younger age, documented to account for up to 30% in adults under 40 years of age and up to 80% in children under 6 (3-5). While most cases of both essential and secondary hypertension are polygenic, a number of disorders have been found to have monogenic etiology. The diagnosis of these hereditary conditions can be challenging, but can also be critical for guiding appropriate care for patients and potentially affected family members.
Here we provide an overview of known hereditary disorders that specifically impact mineralocorticoid action and ultimately cause low renin hypertension. These conditions include familial hyperaldosteronism, defects in steroidogenesis, altered mineralocorticoid receptor activation in the kidney, and changes in the endpoint of mineralocorticoid action, namely the renal epithelial sodium channel (ENaC). These disorders should be considered in the differential diagnosis of young patients presenting with hypertension and hypokalemia, particularly those with a family history of hypertension with hypokalemia. The aim of this overview is to be a useful guide in the work-up of patients with low renin hypertension.
Hereditary causes of primary aldosteronism (PA)
The most common cause of secondary hypertension is PA, which accounts for up to 20% of resistant hypertension and ~8% of all hypertension (6). Although PA is classically characterized by hypokalemia and hypertension, it has become clear that its spectrum extends to normokalemic hypertension as well. The underlying etiology is either a unilateral aldosterone producing adenoma (APA) or bilateral adrenal aldosterone excess, both present in roughly 50% of cases. On diagnostic testing, hypokalemia can be mild or absent, but aldosterone is elevated with suppressed renin. Confirmatory testing includes salt loading, saline infusion, or fludrocortisone application to evaluate for suppression of urinary aldosterone, and, ultimately, adrenal vein sampling for lateralization. In the setting of strong family history of PA and bilateral origin of aldosterone excess, genetic testing for pathogenic variants in genes mentioned below should be considered. Unilateral adrenalectomy is an option in cases of lateralization of aldosterone overproduction; otherwise medical management with mineralocorticoid antagonists is recommended.
Four types of familial hyperaldosteronism have been recognized. Familial hyperaldosteronism type 1, also known as glucocorticoid-remediable aldosteronism (GRA), is an autosomal dominant condition due to the fusion of CYP11B1 and CYP11B2 encoding for 11β-hydroxylase and aldosterone synthase, respectively (7). Transcription of CYP11B1 is usually induced by adrenocorticotropic hormone (ACTH) leading to cortisol synthesis, and transcription of CYP11B2 by angiotensin 2 results in aldosterone synthesis. In the setting of GRA-associated gene fusion, CYP11B2 expression becomes ACTH responsive, resulting in ACTH-induced aldosterone production. Notably, GRA has been associated with increased risk for early hemorrhagic cerebrovascular events (8). In addition to basal plasma aldosterone and renin testing, recommended diagnostic testing includes dexamethasone suppression of aldosterone and genetic testing. The typical protocol for dexamethasone suppression testing is to measure aldosterone before and after administering dexamethasone 0.5 mg every 6 hours for 4 days (9,10), though dexamethasone dosing and duration ranging from 0.75–2.0 mg per day, administered for 2–15 days, have been reported (11). Unlike other forms of PA, glucocorticoids at physiologic and below physiologic dosing have been shown to be an effective therapeutic option, suppressing ACTH and thereby decreasing aldosterone production (12).
Familial hyperaldosteronism types 2, 3, and 4 are not responsive to glucocorticoids. Familial hyperaldosteronism type 2 is clinically indistinguishable from sporadic PA. No notable differences in age of disease onset, plasma renin, serum aldosterone, or potassium levels are seen. Both APAs and bilateral excess aldosterone production are associated with familial hyperaldosteronism type 2 (13-15). It is likely inherited in an autosomal dominant pattern and has been associated with gain-of-function mutations in the CLCN2 gene, encoding for chloride channel protein 2 expressed in adrenal glomerulosa cells (16,17). The penetrance of this genetic disorder is currently unknown, but it is suggested to be incomplete.
Familial hyperaldosteronism type 3 is a rare, highly penetrant, autosomal dominant disorder due to mutations in the potassium channel gene KCNJ5 (18). The initial cases described were in a single family, with multiple members presenting with refractory hypertension and bilateral adrenal gland hyperplasia (19). All family members ultimately required bilateral adrenalectomy to manage their hypertension. Most of the subsequent reported cases of KCNJ5 pathogenic variants similarly have childhood onset severe hypertension, associated with hypokalemia, aldosterone levels greater than 100 ng/dL and low renin activity less than 1.0 ng/mL/h, ultimately requiring either life-long mineralocorticoid antagonist therapy or bilateral adrenalectomy for management (19-21); adrenal pathology often shows hyperplasia of the zona glomerulosa or fasciculata. However, milder phenotypes and ACTH-dependent aldosterone overproduction have also been reported (22,23).
Familial hyperaldosteronism type 4 is a rare condition due to mutations in CACNA1H, encoding for calcium channels expressed in adrenal glomerulosa (24,25). Similar to familial hyperaldosteronism type 2, type 4 is typically indistinguishable from sporadic PA in clinical presentation, and may present with normal appearing adrenal glands, single adenoma, or bilateral hyperplasia.
Another gene with variants associated with PA, which is not classified as a type of familial hyperaldosteronism, is CACNA1D. Pathogenic variants in this gene, encoding for calcium channel Cav1.3, can lower depolarization potential and impair channel inactivation; the resulting increase in calcium influx is thought to stimulate aldosterone production and glomerulosa cell proliferation (26,27). Few individuals with CACNA1D mutations have been described, and notably present with a syndrome of PA, seizures and neuromuscular abnormalities (26,28).
Defects in steroid hormone synthesis
Congenital adrenal hyperplasia (CAH) encompasses a group of inherited disorders resulting in defects in cortisol synthesis. While the most common form of CAH, 21-hydroxylase deficiency, is associated with mineralocorticoid deficiency, 11β-hydroxylase deficiency and 17α-hydroxylase deficiency are the two types of CAH associated with mineralocorticoid excess, resulting in hypertension.
11β-hydroxylase deficiency
11β-hydroxylase deficiency is the second most common form of CAH, accounting for approximately 5% of CAH cases in some series (29). 11β-hydroxylase deficiency is caused by autosomal recessive mutations in CYP11B1, of which more than 20 pathogenic variants have been identified (30-34). CYP11B1 encodes for the function of 11β-hydroxylase, which converts 11-deoxycortisol to cortisol; the expression of the CYP11B1 gene in the zona fasciculate is regulated by ACTH. Reduced activity of 11-hydroxylase causes decreased production of cortisol, which results in elevation of ACTH. This elevation then causes accumulation of steroid precursors, deoxycorticosterone (DOC) and 11-deoxycortisol, as well as overproduction of androgens. The mineralocorticoid activity of DOC and related metabolites causes salt retention, hypertension, and hypokalemia. The clinical presentation of 11β-hydroxylase deficiency can be variable (35), depending on degree of enzyme deficiency: classic 11β-hydroxylase deficiency presents with ambiguous genitalia in genetic females and features of hyperandrogenism in both sexes, while these symptoms may be mild in the non-classic form (36). Hypertension is seen in approximately two-thirds of individuals with the classic form of 11β-hydroxylase deficiency (37) and often within the first year of life, but is not seen in those with the non-classic form. In male patients with 11β-hydroxylase deficiency, hypertension and hypokalemia alone can be the clinical presentation. Diagnosis is made by identifying elevated basal 11-deoxycortisol or elevated ACTH-stimulated 11-deoxycortisol levels, the latter reaching 3-5 times the upper limit of normal in non-classic cases and much higher in classic 11β-hydroxylase deficiency (36). The clinical mineralocorticoid excess is entirely due to DOC, and aldosterone is usually low or absent due to the low renin state. Treatment involves use of glucocorticoids to suppress ACTH-stimulated androgen overproduction and/or mineralocorticoid receptor antagonists. Spironolactone can be used in females for both mineralocorticoid as well as androgen antagonism.
17α-hydroxylase deficiency
17α-hydroxylase deficiency is a rare form of CAH caused by mutations in CYP17A1, encoding for enzyme activity of both 17α-hydroxylase and 17,20-lyase (38,39). In most affected individuals, both enzyme activities are impacted, but deficiency of 17,20-lyase with intact 17α-hydroxylase has also been reported (40,41). More than 100 mutations have been identified in the CYP17A1 gene in association with 17α-hydroxylase deficiency (42-44). 17α-hydroxylase converts progesterone and pregnenolone to 17OH-progesterone and 17OH-pregnenolone, respectively (45). Subsequently, 17,20-lyase converts 21-carbon 17-hydroxysteroids to 19-carbon precursors of sex steroids. 17α-hydroxylase deficiency therefore decreases production of cortisol and sex hormones, resulting in elevation of ACTH and shunting of precursors to DOC and corticosterone production. Subsequent volume expansion and hypokalemia then suppress renin and aldosterone production. Adrenal crises are rare in the presence of excess DOC and corticosterone. Classic clinical presentation is characterized by hypertension and absence of secondary sexual characteristics in an adolescent; both genetically XX and XY individuals with severe complete deficiency can have female external genitalia and primary amenorrhea (45,46). In partial 17α-hydroxylase deficiency, hypertension or hypokalemia may not be present, sexual development is impaired to a lesser extent, and XY individuals may be identified to have ambiguous genitalia in infancy (47). Diagnosis is made when basal or ACTH-stimulated DOC and corticosterone are elevated along with low cortisol, androgens, and estrogens (48). Management of 17α-hydroxylase deficiency includes mineralocorticoid antagonism, physiologic dosing of glucocorticoids and sex hormones to achieve the desired gender and secondary sexual characteristics. In individuals maintaining female phenotype, spironolactone is the drug of choice.
Inappropriate activation of the mineralocorticoid receptor
In addition to overproduction of mineralocorticoids, the overactivation of mineralocorticoid receptors can also result in low renin hypertension. Syndrome of apparent mineralocorticoid excess and constitutive activation of the mineralocorticoid receptor are two such disorders in which mineralocorticoid receptors are inappropriately activated in the absence of mineralocorticoid excess, with associated suppression of aldosterone and renin production.
Apparent mineralocorticoid excess
Under normal function of the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD), the vast majority of cortisol is converted peripherally to cortisone in tissues where this enzyme is expressed (49). Cortisol binds as avidly to mineralocorticoid receptors as aldosterone does, while cortisone does not. In the setting of 11β-HSD deficiency, cortisol, present in the plasma at a concentration approximately 1,000 times higher than aldosterone, binds mineralocorticoid receptors, resulting in the clinical presentation of the aptly named syndrome of apparent mineralocorticoid excess (50,51). A rare disorder with less than 100 cases reported in the literature (52), apparent mineralocorticoid excess is caused by autosomal recessive mutations in HSD11B2 (53). Patients present in childhood with hypertension, poor growth, and polydipsia. Initial lab findings include hypokalemia with low renin, low aldosterone, and low levels of other mineralocorticoids. Patients may also have metabolic alkalosis. Presentation may be mild in instances of partial residual enzyme function (54). On diagnostic testing, the 24-hour urinary cortisol to urinary free cortisone ratio is high (50). A number of genetic testing options are available. Treatment of apparent mineralocorticoid excess requires use of mineralocorticoid antagonists. Spironolactone may be effective initially, but its effectiveness can wane. Cortisol suppression with small doses of dexamethasone (e.g., 0.75 mg) can be considered in refractory cases. The clinical course can also be complicated by hypercalciuria or nephrocalcinosis, though the etiology of this is not well studied. It is thought to be related to chronic hypokalemia, as seen in Bartter syndrome, and can be treated with thiazide diuretics (55,56). Cure of apparent mineralocorticoid excess after renal transplantation has been reported (57).
Constitutive activation of the mineralocorticoid receptor (Geller syndrome)
In vitro studies have shown that mineralocorticoid receptors are specifically activated by some steroids such as aldosterone, antagonized by other steroids such as progesterone, or have no response to steroids such estradiol and testosterone (58). Geller et al. (58) identified in a 15-year-old boy with severe hypertension, a single missense mutation in the mineralocorticoid receptor gene that resulted in gain-of-function variant and constitutive activation of the mineralocorticoid receptor. The condition was found to have an autosomal dominant inheritance pattern, with 11 of 23 relatives studied also carrying diagnoses of severe hypertension before age 20. The individuals had low serum renin and aldosterone. Two affected female family members had notable hypertension during pregnancy, without edema or proteinuria seen in preeclampsia. Serum aldosterone was undetectably low, while it is typically elevated in pregnancy (59). The cause of worsening hypertension during pregnancy is thought to be due to mutant mineralocorticoid receptor activation by progesterone. Hypertension is expected to improve after delivery. Of note, spironolactone was found to activate the mutant mineralocorticoid receptors in vitro, so use of other antihypertensive medications is recommended. The syndrome of constitutive activation of mineralocorticoid receptors is an extremely rare syndrome and no additional cases have been described following the initial publication by Geller et al.
Other low renin states
The final target of mineralocorticoid action is sodium channels of the kidney. Both Liddle syndrome and pseudohypoaldosteronism type 2 (PHA2) are disorders impacting the function of the ENaC of the distal nephron; the resulting increase in renal sodium reabsorption precipitates volume overload, suppressed renin, and electrolyte abnormalities.
Liddle syndrome
Liddle et al. (60) described a family with multiple teenage siblings who presented with hypertension, hypokalemia and metabolic alkalosis. The disorder was also termed pseudoaldosteronism, given its clinical similarity with PA, but the individuals’ renin and aldosterone were both low, even when challenged with low sodium diets. On follow-up, the proband case developed renal disease and underwent kidney transplant, which resolved her disorder—and indicated that her condition was due to a primary renal, rather than mineralocorticoid issue (61). Liddle syndrome was ultimately found to be due to autosomal dominant mutations in SCNN1B and SCNN1G, encoding for subunits of the ENaC of the distal nephron, which results in constitutive activation of the channel (62). Physiologically, ENaC expression is the endpoint of mineralocorticoid action, leading to increased sodium absorption, as would be appropriate in the setting of volume depletion. Constitutive ENaC activation in Liddle syndrome causes excess sodium resorption in the kidney leading to hypertension with suppressed renin and aldosterone. Typically, patients present in childhood, but sometimes reach early adulthood before diagnosis. More than 20 pathogenic variants in the β and γ subunits of the ENaC have been identified (62-66). The diagnosis is confirmed with genetic testing for variants in SCNN1B and SCNN1G. The disorder is responsive to low salt diet and medications that block ENaC activity, such as amiloride and triamterene. As ENaC expression is not dependent on mineralocorticoid activation, mineralocorticoid antagonists such as spironolactone are ineffective (67,68).
PHA2 (Gordon syndrome)
Gordon et al. (69) first described a case of hypertension and hyperkalemia in a 10-year-old girl without a history of renal impairment. The observation of hyperkalemia led to the classification of this syndrome as PHA2 and differentiates it from PA. PHA2 is a distinct entity from PHA types 1A and 1B, which result in loss of function of the ENaC channel and are accompanied by a clinical constellation resembling mineralocorticoid deficiency, with salt wasting, severe hyperkalemia, and hypotension. PHA2 has been associated with autosomal dominant mutations in the WNK1 and WNK4 genes, which are involved in the inhibition of the sodium chloride cotransporter (70-72); the mutations result in increased sodium reabsorption in the distal convoluted tubule, with associated downstream effects on ENaC and renal outer medullary potassium channel that ultimately cause hyperkalemia and hyperchloremic metabolic acidosis. Mutations in the KLHL3 and CUL3 genes, encoding proteins that degrade WNK kinases, have also been associated with PHA2 (73,74). Affected individuals present in adolescence or young adulthood with normal to mildly elevated aldosterone levels; genetic testing confirms the diagnosis. The condition is responsive to low salt diet and thiazide diuretics, which act on the sodium-chloride cotransporter (75).
Summary and conclusion
Advances in genetic studies have allowed progressive elucidation of hereditary causes of hypertension. In particular, genetic testing has helped to characterize a number of low-renin hypertension states that can be challenging to differentiate from PA. While many of these conditions are rare, their management is considerably different from polygenic essential hypertension. Therefore, diagnosis of these disorders is essential for effective treatment of affected patients, as well as guiding testing for family members.
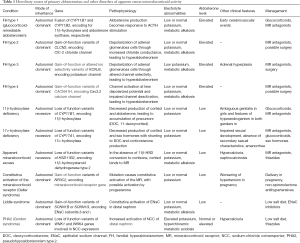
Full table
Acknowledgments
The authors would like to thank Richard J. Auchus for his expertise and critical reading of the manuscript.
Funding: Part of this work was supported by 5 R01 HL130106-05.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Puar TH, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J 2016;57:228-32. [Crossref] [PubMed]
- Fountoulakis S, Tsatsoulis A. Molecular genetic aspects and pathophysiology of endocrine hypertension. Hormones (Athens) 2006;5:90-106. [Crossref] [PubMed]
- Baracco R, Kapur G, Mattoo T, et al. Prediction of primary vs secondary hypertension in children. J Clin Hypertens (Greenwich) 2012;14:316-21. [Crossref] [PubMed]
- Flynn J, Zhang Y, Solar-Yohay S, et al. Clinical and demographic characteristics of children with hypertension. Hypertension 2012;60:1047-54. [Crossref] [PubMed]
- Camelli S, Bobrie G, Postel-Vinay N, et al. Prevalence of secondary hypertension in young hypertensive adults. J Hypertens 2015;33:e47. [Crossref]
- Byrd JB, Turcu AF, Auchus RJ. Primary Aldosteronism. Circulation 2018;138:823-35. [Crossref] [PubMed]
- Lifton RP, Dluhy RG, Powers M, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992;355:262-5. [Crossref] [PubMed]
- Litchfield WR, Anderson BF, Weiss RJ, et al. Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension 1998;31:445-50. [Crossref] [PubMed]
- Stowasser M, Bachmann AW, Jonsson JR, et al. Clinical, biochemical and genetic approaches to the detection of familial hyperaldosteronism type I. J Hypertens 1995;13:1610-3. [PubMed]
- Mulatero P, Veglio F, Pilon C, et al. Diagnosis of Glucocorticoid-Remediable Aldosteronism in Primary Aldosteronism: Aldosterone Response to Dexamethasone and Long Polymerase Chain Reaction for Chimeric Gene. J Clin Endocrinol Metab 1998;83:2573-5. [Crossref] [PubMed]
- Litchfield WR, New MI, Coolidge C, et al. Evaluation of the Dexamethasone Suppression Test for the Diagnosis of Glucocorticoid-Remediable Aldosteronism. J Clin Endocrinol Metab 1997;82:3570-3. [PubMed]
- Stowasser M, Bachmann AW, Huggard PR, et al. Treatment of familial hyperaldosteronism type I: only partial suppression of adrenocorticotropin required to correct hypertension. J Clin Endocrinol Metab 2000;85:3313-8. [Crossref] [PubMed]
- Stowasser M, Gordon RD, Tunny TJ, et al. Familial Hyperaldosteronism Type Ii: Five Families with a New Variety of Primary Aldosteronism. Clin Exp Pharmacol Physiol 1992;19:319-22. [Crossref] [PubMed]
- Torpy DJ, Gordon RD, Lin JP, et al. Familial Hyperaldosteronism Type II: Description of a Large Kindred and Exclusion of the Aldosterone Synthase (CYP11B2) Gene. J Clin Endocrinol Metab 1998;83:3214-8. [PubMed]
- Sukor N, Mulatero P, Gordon RD, et al. Further evidence for linkage of familial hyperaldosteronism type II at chromosome 7p22 in Italian as well as Australian and South American families. J Hypertens 2008;26:1577-82. [Crossref] [PubMed]
- Scholl UI, Stölting G, Schewe J, Thiel A, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet 2018;50:349-54. [Crossref] [PubMed]
- Fernandes-Rosa FL, Daniil G, Orozco IJ, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet 2018;50:355-61. [Crossref] [PubMed]
- Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011;331:768-72. [Crossref] [PubMed]
- Geller DS, Zhang J, Wisgerhof MV, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 2008;93:3117-23. [Crossref] [PubMed]
- Scholl UI, Nelson-Williams C, Yue P, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A 2012;109:2533-8. [Crossref] [PubMed]
- Charmandari E, Sertedaki A, Kino T, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab 2012;97:E1532-9. [Crossref] [PubMed]
- Adachi M, Muroya K, Asakura Y, et al. Discordant Genotype-Phenotype Correlation in Familial Hyperaldosteronism Type III with KCNJ5 Gene Mutation: A Patient Report and Review of the Literature. Horm Res Paediatr 2014;82:138-42. [Crossref] [PubMed]
- Sertedaki A, Markou A, Vlachakis D, et al. Functional characterization of two novel germline mutations of the KCNJ5 gene in hypertensive patients without primary aldosteronism but with ACTH-dependent aldosterone hypersecretion. Clin Endocrinol (Oxf) 2016;85:845-51. [Crossref] [PubMed]
- Scholl UI, Stölting G, Nelson-Williams C, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife 2015;4:e06315. [Crossref] [PubMed]
- Daniil G, Fernandes-Rosa FL, Chemin J, et al. CACNA1H Mutations Are Associated With Different Forms of Primary Aldosteronism. EBioMedicine 2016;13:225-36. [Crossref] [PubMed]
- Scholl UI, Goh G, Stölting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013;45:1050-4. [Crossref] [PubMed]
- Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 2013;45:1055-60. [Crossref] [PubMed]
- Semenova NA, Ryzhkova OR, Strokova TV, et al. The third case report a patient with primary aldosteronism, seizures, and neurologic abnormalities (PASNA) syndrome de novo variant mutations in the CACNA1D gene. Zh Nevrol Psikhiatr Im S S Korsakova 2018;118:49-52. [Crossref] [PubMed]
- Werder EA, Siebenmann RE, Knorr-Murset G, et al. The incidence of congenital adrenal hyperplasia in Switzerland: A survey of patients born in 1960 to 1974. Helv Paediatr Acta 1980;35:5-11. [PubMed]
- Alqahtani MA, Shati AA, Zou M, et al. A Novel Mutation in the CYP11B1 Gene Causes Steroid 11β-Hydroxylase Deficient Congenital Adrenal Hyperplasia with Reversible Cardiomyopathy. Int J Endocrinol 2015;2015:595164. [Crossref] [PubMed]
- Curnow KM, Slutsker L, Vitek J, et al. Mutations in the CYP11B1 gene causing congenital adrenal hyperplasia and hypertension cluster in exons 6, 7, and 8. Proc Natl Acad Sci U S A 1993;90:4552-6. [Crossref] [PubMed]
- Geley S, Kapelari K, Jöhrer K, et al. CYP11B1 mutations causing congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. J Clin Endocrinol Metab 1996;81:2896-901. [PubMed]
- Naiki Y, Kawamoto T, Mitsuuchi Y, et al. A nonsense mutation (TGG [Trp116]-->TAG [Stop]) in CYP11B1 causes steroid 11 beta-hydroxylase deficiency. J Clin Endocrinol Metab 1993;77:1677-82. [PubMed]
- Helmberg A, Ausserer B, Kofler R. Frame shift by insertion of 2 basepairs in codon 394 of CYP11B1 causes congenital adrenal hyperplasia due to steroid 11 beta-hydroxylase deficiency. J Clin Endocrinol Metab 1992;75:1278-81. [PubMed]
- Zachmann M, Tassinari D, Prader A. Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients. J Clin Endocrinol Metab 1983;56:222-9. [Crossref] [PubMed]
- Joehrer K, Geley S, Strasser-Wozak EMC, et al. CYP11B1 Mutations Causing Non-Classic Adrenal Hyperplasia due to 11β-Hydroxylase Deficiency. Hum Mol Genet 1997;6:1829-34. [Crossref] [PubMed]
- Rösler A, Leiberman E, Cohen T. High frequency of congenital adrenal hyperplasia (classic 11β-hydroxylase deficiency) among Jews from Morocco. Am J Med Genet 1992;42:827-34. [Crossref] [PubMed]
- Sparkes RS, Klisak I, Miller WL. Regional mapping of genes encoding human steroidogenic enzymes: P450scc to 15q23-q24, adrenodoxin to 11q22; adrenodoxin reductase to 17q24-q25; and P450c17 to 10q24-q25. DNA Cell Biol 1991;10:359-65. [Crossref] [PubMed]
- Fan YS, Sasi R, Lee C, et al. Localization of the human CYP17 gene (cytochrome P450(17 alpha)) to 10q24.3 by fluorescence in situ hybridization and simultaneous chromosome banding. Genomics 1992;14:1110-1. [Crossref] [PubMed]
- Geller DH, Auchus RJ, Mendonça BB, et al. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet 1997;17:201-5. [Crossref] [PubMed]
- Zachmann M, Völlmin JA, Hamilton W, et al. Steroid 17,20-desmolase deficiency: a new cause of male pseudohermaphroditism. Clin Endocrinol (Oxf) 1972;1:369-85. [Crossref] [PubMed]
- Fardella CE, Hum DW, Homoki J, et al. Point mutation of Arg440 to His in cytochrome P450c17 causes severe 17 alpha-hydroxylase deficiency. J Clin Endocrinol Metab 1994;79:160-4. [PubMed]
- Yanase T. 17α-Hydroxylase/17,20-lyase defects. J Steroid Biochem Mol Biol 1995;53:153-7. [Crossref] [PubMed]
- Zhang M, Sun S, Liu Y, et al. New, recurrent, and prevalent mutations: Clinical and molecular characterization of 26 Chinese patients with 17alpha-hydroxylase/17,20-lyase deficiency. J Steroid Biochem Mol Biol 2015;150:11-6. [Crossref] [PubMed]
- Biglieri EG, Herron MA, Brust N. 17-hydroxylation deficiency in man. J Clin Invest 1966;45:1946-54. [Crossref] [PubMed]
- Heremans GF, Moolenaar AJ, van Gelderen HH. Female phenotype in a male child due to 17-alpha-hydroxylase deficiency. Arch Dis Child 1976;51:721-3. [Crossref] [PubMed]
- Rubtsov P, Nizhnik A, Dedov I, et al. Partial deficiency of 17α-hydroxylase/17,20-lyase caused by a novel missense mutation in the canonical cytochrome heme-interacting motif. Eur J Endocrinol 2015;172:K19-25. [Crossref] [PubMed]
- Kater CE, Biglieri EG. Disorders of steroid 17 alpha-hydroxylase deficiency. Endocrinol Metab Clin North Am 1994;23:341-57. [Crossref] [PubMed]
- Edwards CR, Stewart PM, Burt D, et al. Localisation of 11 beta-hydroxysteroid dehydrogenase--tissue specific protector of the mineralocorticoid receptor. Lancet 1988;2:986-9. [Crossref] [PubMed]
- Ulick S, Kodama T, Gunczier P, et al. A Syndrome of Apparent Mineralocorticoid Excess Associated with Defects in the Peripheral Metabolism of Cortisol*. J Clin Endocrinol Metab 1979;49:757-64. [Crossref] [PubMed]
- New MI, Levine LS, Biglieri EG, et al. Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension. J Clin Endocrinol Metab 1977;44:924-33. [Crossref] [PubMed]
- Limumpornpetch P, Stewart PM. Apparent Mineralocorticoid Excess. In: Huhtaniemi I, Martini L. editors. Encyclopedia of Endocrine Diseases. 2nd edition. Oxford: Academic Press, 2019:638-43.
- Mune T, Rogerson FM, Nikkilä H, et al. Human hypertension caused by mutations in the kidney isozyme of 11 beta-hydroxysteroid dehydrogenase. Nat Genet 1995;10:394-9. [Crossref] [PubMed]
- Funder JW. Apparent mineralocorticoid excess. J Steroid Biochem Mol Biol 2017;165:151-3. [Crossref] [PubMed]
- White PC, Mune T, Agarwal AK. 11β-Hydroxysteroid Dehydrogenase and the Syndrome of Apparent Mineralocorticoid Excess. Endocr Rev 1997;18:135-56. [PubMed]
- Simon DB, Karet FE, Hamdan JM, et al. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na–K–2CI cotransporter NKCC2. Nat Genet 1996;13:183-8. [Crossref] [PubMed]
- Razzaghy-Azar M, Yau M, Khattab A, et al. Apparent mineralocorticoid excess and the long term treatment of genetic hypertension. J Steroid Biochem Mol Biol 2017;165:145-50. [Crossref] [PubMed]
- Geller DS, Farhi A, Pinkerton N, et al. Activating Mineralocorticoid Receptor Mutation in Hypertension Exacerbated by Pregnancy. Science 2000;289:119-23. [Crossref] [PubMed]
- Elsheikh A, Creatsas G, Mastorakos G, et al. The renin-aldosterone system during normal and hypertensive pregnancy. Arch Gynecol Obstet 2001;264:182-5. [Crossref] [PubMed]
- Liddle G, Bledsoe T, Coppage WS. A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians 1963;76:199-213.
- Botero-Velez M, Curtis JJ, Warnock DG. Liddle’s Syndrome Revisited – A Disorder of Sodium Reabsorption in the Distal Tubule. N Engl J Med 1994;330:178-81. [Crossref] [PubMed]
- Shimkets RA, Warnock DG, Bositis CM, et al. Liddle’s syndrome: Heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell 1994;79:407-14. [Crossref] [PubMed]
- Hansson JH, Nelson-Williams C, Suzuki H, et al. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 1995;11:76-82. [Crossref] [PubMed]
- Jackson SN, Williams B, Houtman P, et al. The diagnosis of Liddle syndrome by identification of a mutation in the beta subunit of the epithelial sodium channel. J Med Genet 1998;35:510-2. [Crossref] [PubMed]
- Gao L, Wang L, Liu Y, et al. A family with Liddle syndrome caused by a novel missense mutation in the PY motif of the beta-subunit of the epithelial sodium channel. J Pediatr 2013;162:166-70. [Crossref] [PubMed]
- Uehara Y, Sasaguri M, Kinoshita A, et al. Genetic analysis of the epithelial sodium channel in Liddle’s syndrome. J Hypertens 1998;16:1131-5. [Crossref] [PubMed]
- Wang C, Chan TK, Yeung RT, et al. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981;52:1027-32. [Crossref] [PubMed]
- Nakada T, Koike H, Akiya T, et al. Liddle’s syndrome, an uncommon form of hyporeninemic hypoaldosteronism: functional and histopathological studies. J Urol 1987;137:636-40. [Crossref] [PubMed]
- Gordon RD, Geddes RA, Pawsey CG, et al. Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas Ann Med 1970;19:287-94. [Crossref] [PubMed]
- Wilson FH, Disse-Nicodème S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science 2001;293:1107-12. [Crossref] [PubMed]
- Lalioti MD, Zhang J, Volkman HM, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 2006;38:1124-32. [Crossref] [PubMed]
- Yang C-L, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 2007;117:3403-11. [Crossref] [PubMed]
- Boyden LM, Choi M, Choate KA, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 2012;482:98-102. [Crossref] [PubMed]
- Yoshida S, Araki Y, Mori T, et al. Decreased KLHL3 expression is involved in the pathogenesis of pseudohypoaldosteronism type II caused by cullin 3 mutation in vivo. Clin Exp Nephrol 2018;22:1251-7. [Crossref] [PubMed]
- Gordon RD, Hodsman GP. The Syndrome of Hypertension and Hyperkalaemia without Renal Failure: Long Term Correction by Thiazide Diuretic. Scott Med J 1986;31:43-4. [Crossref] [PubMed]


