Histologic evaluation of sentinel and non-sentinel axillary lymph nodes in breast cancer by multilevel sectioning and predictors of non-sentinel metastasis
Introduction
Axillary node status is one of the most important prognostic indicators in breast cancer and is of particular value in considering the choice of adjuvant therapy. Axillary node dissection has long been the standard procedure for determining the nodal stage in breast cancer but its complications can be disabling. During the past three decades, the surgical management of breast cancer has changed significantly. Several large multicenter trials have established breast conservation procedure with the advent of sentinel lymph node (SLN) biopsy (1-5). The SLN biopsy is rapidly gaining popularity as a staging procedure for breast cancer. It enables selective targeting of the first tumor draining lymph nodes, where the initial metastasis will form. Conceptually, a negative SLN predicts the absence of tumor metastasis in the other regional lymph nodes with a high degree of accuracy. Multiple studies showed that SLN examination had a sensitivity of 83.4-100.0% for the detection of axillary lymph node disease and the accuracy in determining axillary lymph node status in comparison with standard axillary lymph node dissection was 92-96.4% (3,5-9).
At present, the sentinel node biopsy is widely used as a definite staging procedure of breast cancer. It has been shown through numerous studies that routine histological examination of dissected nodes may be inadequate depending on the thoroughness of examination. In a number of large studies using different histopathologic techniques, the SLN false-negative rate has varied between 0-11% (2,3,6,8,9). Many investigators have reported finding micrometastases that were not detected by routine sectioning of lymph nodes, but were identified by multiple sectioning and additional immunohistochemistry staining (1,4,6,10-16). However, the optimal pathologic examination of the SLN has not yet been determined. The method used is individualized in each hospital. The study of SLN using isosulfan blue as the dye in Siriraj Hospital was first established by Ratanawichitrasin et al. in 1998 (8). The SLN and NSLN simultaneous removals were designed to assess the accuracy in prediction of the state of the axilla. The intensive histologic study of these samples was planned to assess the validity of SLN biopsy, compare the results using standard examination and multilevel sectioning, identify the accurate metastatic size, and determine the predictive factors of metastasis in NSLN when the SLN is positive.
Materials and methods
This study has been approved by the institutional ethic committee (IEC), approval number Si 197/2005. It was a retrospective study using subjects from the study of “Lymphatic mapping and SLN biopsy in breast cancer patients” by Ratanawichitrasin et al., Department of Surgery, Siriraj Hospital, in 1998-2002. There were 215 patients in an early stage (T0-2, tumor size mostly 0.2-2 mm) and macrometastasis (>2 mm), according to the pathological staging of involved axillary lymph node of the AJCC staging, 6th edition (17).
Statistical analysis
An independent sample t-test was used to test the difference in quantitative variables between subjects with and without metastasis. Fisher’s exact test was employed to test the association between metastasis and unordered or binary qualitative variable, e.g., tumor size (≤2, >2 cm), location (upper outer, others). Linear-by-linear association test was used to assess the relationship between metastasis and ordered qualitative variable, e.g., six tumor size groups, location (others, inner, outer), three histologic grades. Independent variables with univariable P-value of less than 0.2 were included in a multiple logistic regression model to assess the effect of each variable on axillary lymph node metastasis after adjusting for effect of the others. For moderate sample size, 95% CI of adjusted OR was computed by profile likelihood method instead of maximum likelihood method. With small sample size, P-value, adjusted OR, 95% CI of adjusted OR from logistic regression were obtained from exact method. Statistical data analyses were performed using SAS 8.1 and StatXact 6. An exact 2-sided P-value of less than 0.05 was considered statistical significance.
Results
Of 195 patients studied, the mean age and tumor size was 48.9 years (range: 28-78 years) and 2.31 cm (SD =1.12, range =0.08-6.5), respectively. A total of 185 patients (95%) had invasive carcinomas with 166 patients (85%) having invasive ductal lesions, 46% of which were moderately differentiated. The other ten patients had carcinoma in situ. The number of the SLNs and NSLNs in each patient ranged from 1-8 nodes (mean =3, median =2) and 1-53 nodes (mean =21, median =20), respectively.
Standard HE and multilevel study
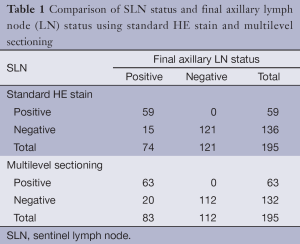
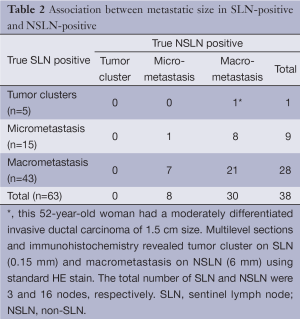
The standard HE stain on the initial sections identified 59 (30.2%) of 195 cases with SLN metastases. Among 136 SLN-negative cases, 15 cases showed NSLN metastasis (Table 1). Multilevel sections and immunohistochemistry on negative SLNs revealed 4 additional cases of SLN-positive (6.3% of all 63 patients with true positive SLN). Five NSLN-negative cases turned to be positive; 2 had tumor clusters and 3 had micrometastases (Table 1). The sensitivity of the SLN technique using standard method was 79.7% (95% CI: 68.8%, 88.2%) compared to 75.9% (95% CI: 65.3%, 84.6%) using multilevel HE study. The specificity of the SLN technique for both standard and multilevel method was 100% (95% CI: 97%, 100%). The positive predictive value was 100% (63/63). The negative predictive value of standard method and multilevel HE study were 88.9% and 84.8%, respectively. Concordance between the sentinel and the final pathologic lymph node status was 92.3% using standard method and 89.7% using multilevel HE study. A false-negative rate increased from 20.3% (15/74) for standard method to 24.1% (20/83) for multilevel HE study. Multilevel sectioning could detect positive axillary lymph node 10.8% (9/83) more than standard HE stain. Of all 63 SLN-positive cases, 5 (7.9%) were tumor cluster, 15 (23.8%) micrometastasis and the remaining 43 (68.2%) macrometastasis. Association between the size of the positive-SLN and positive-NSLN was shown in Table 2. The mean primary tumor size in SLN cases with tumor clusters, micrometastasis and macrometastasis were 1.7, 2.2 and 2.9 cm. respectively.
Full table
Full table
Factors determining axillary lymph node metastasis
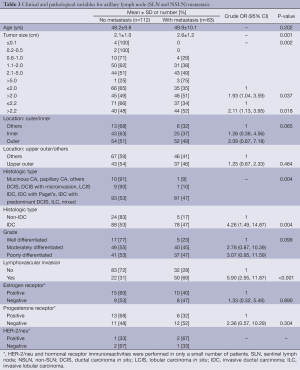
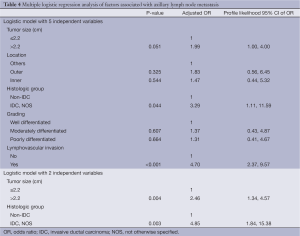
Univariable analysis showed that factors significantly associated with axillary lymph node metastasis were tumor size, histologic type [invasive ductal carcinoma, not otherwise specified (IDC, NOS) and non IDC, NOS] and lymphovascular space invasion (Table 3). Patients with tumor size greater than 2.2 cm had 2.11 higher risk of metastasis than those with tumor size less than or equal to 2.2 cm. Histologic type of IDC, NOS and LVI were associated with a higher risk of metastasis with crude odds ratios of 4.26 and 5.90, respectively. Location (inner/outer/others) and primary tumor grade (well/moderately/poorly differentiated) seemed to be related to metastasis but they were not statistically significant (P=0.065, 0.099, respectively). There was no statistically significant difference in age, ER and PR between patients with and without metastasis. A multiple logistic regression model was fitted using five independent variables with P-value of less than 0.2 from univariable analysis (Table 4). Analysis revealed three statistically significant factors for metastasis: tumor size of >2.2 cm (OR =1.99, P=0.051), histologic type of IDC, NOS (OR =3.29, P=0.044) and LVI (OR =4.70, PTable 4 revealed another multiple logistic model with only two independent variables of tumor size of >2.2 cm. and histologic type of IDC, NOS due to their availability in cytological or biopsy reports. Analysis showed adjusted OR of 2.46 (P=0.004) and 4.85 (P=0.003) for tumor size >2.2 cm and histologic type of IDC, NOS respectively.
Full table
Full table
Univariable analysis of predictive factors for NSLN metastasis (after SLN metastasis) based on standard HE staining group (Table 5) demonstrated that outer (vs. inner) location and perinodal invasion of SLN seemed to be related to metastasis with OR of 3.29 (P=0.072) and 3.24 (P=0.062), respectively. Due to small sample size (n=32 and 27 for with and without metastasis, respectively), a multiple logistic regression model was fitted using exact method with only three independent variables, i.e., location (inner/outer), grade (moderate/poor) and perinodal invasion of SLN (no/yes). Result showed no statistically significant predictors for metastasis (Table 6).
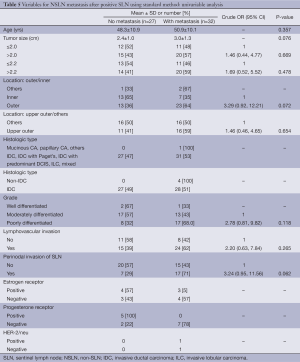
Full table

Full table
Table 7 displayed factors associated with NSLN metastasis based on multilevel sectioning. Outer location significantly increased the risk of metastasis (OR =2.66, P=0.040) compared to the inner location. LVI and perinodal invasion seemed to increase the risk of metastasis as well with OR of 3.15 (P=0.058) and 3.32 (P=0.056) respectively. An exact multiple logistic regression analysis on three independent variables, i.e., location (inner/outer), LVI (no/yes) and perinodal invasion of SLN (no/yes) demonstrated that only outer location increased the risk with adjusted OR of 3.90, P=0.036 (Table 8).
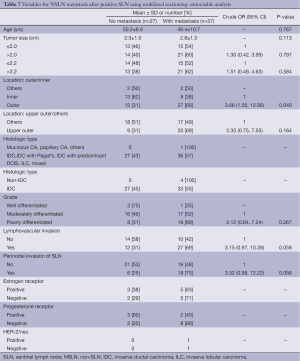
Full table
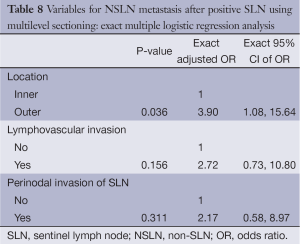
Full table
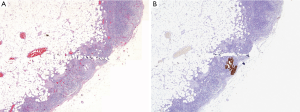
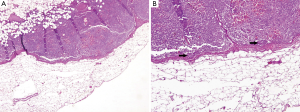
IHC technique increased detection of metastatic deposits. Eight cases were detected by IHC (four cases in SLN and four in NSLN) but not by initial examination of the corresponding HE slides using 4× objective (Figure 1A,B). Among these eight cases, three were tumor clusters (range, 0.1-0.15 mm; Figure 2A,B) and the other five were micrometastases (range, 0.25-1 mm). There was only one case that the IHC identified the metastatic deposit without tumor in HE section.
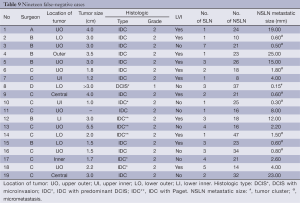
From the 19 false negative SLN almost all had the primary tumors in the upper outer location, histologic type of IDC, NOS and moderate grade (Table 9). The average primary tumor size was 2.6 cm (range, 1-5.5 cm) and numbers of the SLN and NSLN were 2.5 nodes (range, 1-7 nodes) and 22.8 nodes (range, 8-47 nodes), respectively. The metastatic lesions comprised ten macrometastases, eight micrometastases and one tumor cluster. Eleven cases were performed by one surgeon. No recurrent tumor following initial operations was found during the average three-year follow-up period (4.8 months-5.7 years). Among the 19 false negative cases, seven patients who have more than ten years follow up still are free of recurrent disease.
Full table
Discussion
Previous investigations on SLN have been shown to accurately reflect the presence or absence of metastases in the axilla in patients with breast cancer. The incidence of 32.3% positive SLN in this study was within the range of 21.3-46% as studied by others (2,6-9,15). The patients in this study comprised a little more T and N staging since it was an initial study intended to perform dissection of all axillary lymph nodes. Multiple studies showed that SLN examination had a sensitivity of 83.4-100% for the detection of axillary lymph node disease (3,5-9). In a number of large studies using different histopathologic techniques, the SLN false-negative rates varied between 0-11% (2,3,6,8,9). In the present study, the SLN examination had a sensitivity of 75.9%, and a false-negative rate of 24.1%. These results are different from others as the sensitivity was lower and the false negative rate was quite high. As mentioned above, this study was a retrospective study using the same population from the initial study (learning curve) of SLN biopsy in Siriraj Hospital. The SLN identification rate varied depending upon the experience of the surgeons and the techniques used. Most investigators reported that learning these techniques required time and experience (1,7,18). Another reason which might affect the detection of SLN was the technique used, we performed peritumoral dye injection only. Combined dye and radioisotope injection techniques used intraoperatively has been addressed to be superior to using dye alone by comparing the false-negative rate which was higher in the dye alone group (21% versus 2.8%) (19). In addition, variability of lymph flow and rerouting of lymphatic flow by tumor blockage of the lymph node could also affect the false negative rate (1,18).
The 6.3% increased detection of metastases in SLN on multilevel sectioning and IHC was similar to those studied by others which ranged from 3-20% (6,11,12,14,15). In our series with a rather high false-negative rate and a small number of studied cases, the percentage of the SLNs with tumor cluster or micrometastasis that had macrometastasis in the NSLNs were quite high (25% and 53%, respectively) in comparison with other study (20). The finding of tumor cluster or micrometastasis in multilevel sectioning and IHC is significant in false negative cases.
Regarding the predictive factors of axillary node metastasis, Olivotto et al. (21) studied the multivariate analysis of 6,052 patients and found that the readily available prognostic factors for axillary lymph node metastases were nodal palpability, tumor size and LVI. In a series by Joseph et al. (22) of 407 patients who underwent successful SLN dissection, 70 patients (17%) had positive SLN findings. The factors in their study that contributed to NSLN metastasis were tumor size and extranodal extension. Significant predictive factors of NSLN metastasis in the series of Abdessalam et al. (23) were LVI, extranodal extension and increasing size of metastatic focus within the SLN. Turner et al. (24) found that the predictive factors correlated with non SLN metastasis were primary tumor size and peritumoral LVI. In a study of 389 patients with positive SLN, Wong et al. (25) found that the likelihood of positive NSLN correlated with increasing tumor size. This result is similar to the study of Chu et al. (26), Reynolds et al. (27), and Kamath et al. (28). In the present study, we found that tumor size and LVI were significant predictors of axillary lymph node metastasis by multivariate analysis. By univariable analysis the outer location of primary tumor and perinodal invasion were significantly associated with the presence of NSLN metastasis after positive SLN; LVI was also a predictor of NSLN status (from the multilevel study only). The findings were similar to the previously mentioned studies with an addition that a significant association between outer location of primary tumor and NSLN metastasis after positive SLN was also found.
For the SLN histologic examination, in order to decrease the metastasis detection error especially in micrometastasis and tumor cluster, we recommend careful examination of the standard HE section using an objective power 10× in screening the SLN and good quality of HE staining is mandatory. The size of metastatic deposits and perinodal invasion should be included in the report. Although multilevel and immunohistochemistry increased the detection of metastasis, all of them were micrometastases and tumor clusters. The study is costly and ensures a workload for the pathologist. To gain the maximal benefit of SLN biopsy, this intensive method may be considered in the patients at high risk for axillary node metastasis, e.g., those who had tumor size more than 2.2 cm, had IDC, NOS (non-papillary or non-mucinous carcinoma), and lymphovascular space invasion, since 19.5% of the patients with these characters had 77.8% metastasis in axillary node according to our series. However, more studies are needed to find a consensus on which patients are the high risk group to perform the intensive study which may also include other more sensitive test and if the multilevel study is considered, a standard protocol should be proposed.
In summary, a multilevel study of SLN and NSLN was performed and compared to the standard HE method. Caution should be used in using SLN biopsy technique with dye peritumoral injection alone especially in the early performing phase as there might be a high false-negative rate. However, the concordance rate of 89.7% confirmed that SLN biopsy is a reliable factor to determine the axillary nodal status.
Identification and pathological study of the SLN play an important role in the SLN procedure for staging of breast cancer. In breast cancer patients with positive SLN, the outer location of the primary tumor, LVI and perinodal extension significantly increased the frequency of additional positive nodes.
Acknowledgements
The work was supported in part by the Siriraj Grant for Resident’s Research Development from Faculty of Medicine Siriraj Hospital, Mahidol University. The authors thank Ame-on Amesombun and Papassorn Terbto for technical assistance and Kanittar Srisook for immunohistochemistry staining. Supported in part by Siriraj Grant for Research Development.
Disclosure: The authors declare no conflict of interest.
References
- Bonnema J, van de Velde CJH. Sentinel lymph node biopsy in breast cancer. Ann Oncol 2002;13:1531-7. [PubMed]
- Carcoforo P, Sortini D, Soliani G, et al. Accuracy and reliability of sentinel node biopsy in patients with breast cancer. Single centre study with long term follow-up. Breast Cancer Res Treat 2006;95:111-6. [PubMed]
- Gill PG. Sentinel lymph node biopsy versus axillary clearance in operable breast cancer: the RACS SNAC trial, a multicenter randomized trial of the Royal Australian College of Surgeons (RACS) Section of Breast Surgery, in collaboration with the National Health and Medical Research Council Clinical Trials Center. Ann Surg Oncol 2004;11:216S-21S. [PubMed]
- Hunt JL, Baloch ZW. LiVolsi VA. Sentinel lymph node evaluation for tumor metastasis. Semin Diagn Pathol 2002;19:263-77. [PubMed]
- Viale G. Overview of sentinel node trials in Europe. Pathol Int 2004;54:S31.
- Pargaonkar AS, Beissner RS, Snyder S, et al. Evaluation of immunohistochemistry and multiple-level sectioning in sentinel lymph nodes from patients with breast cancer. Arch Pathol Lab Med 2003;127:701-5. [PubMed]
- Ratanawichitrasin A, Levy L, Myles J, et al. Experience with lymphatic mapping in breast cancer using isosulfan blue dye. J Womens Health 1998;7:873-7. [PubMed]
- Ratanawichitrasin A, Rojananin S, Bhothisuwan K, et al. Lymphatic mapping with isosulfan blue and sentinel lymph node biopsy for breast cancer patients. Thai J Surg 1999;20:93-6.
- Somwangprasert A, Lijegren G. Sentinel node in breast cancer. Thai J Surg 1998;20:89-92. [PubMed]
- Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. Lancet 1999;354:896-900. [PubMed]
- Cserni G. Histopathologic examination of the sentinel lymph nodes. Breast J 2006;12:S152-6. [PubMed]
- Cserni G. Metastases in axillary sentinel lymph nodes in breast cancer as detected by intensive histopathological work up. J Clin Pathol 1999;52:922-4. [PubMed]
- Scolyer RA, Murali R, McCarthy SW, et al. Pathologic examination of sentinel lymph nodes from melanoma patients. Semin Diagn Pathol 2008;25:100-11. [PubMed]
- Torrenga H, Rahusen FD, Meijer S, et al. Sentinel node investigation in breast cancer: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2001;54:550-2. [PubMed]
- Turner RR, Ollila DW, Stern S, et al. Optimal histopathologic examination of the sentinel lymph node for breast carcinoma staging. Am J Surg Pathol 1999;23:263-7. [PubMed]
- Yared MA, Middleton LP, Smith TL, et al. Recommendations for sentinel lymph node processing in breast cancer. Am J Surg Pathol 2002;26:377-82. [PubMed]
- Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC cancer staging manual. CA Cancer J Clin 2006;56:37-47.
- Tanis PJ, Nieweg OE, Merkus JW, et al. False negative sentinel node procedure established through palpation of the biopsy wound. Eur J Surg Oncol 2000;26:714-5. [PubMed]
- Syme DB, Collins JP, Mann GB. Comparison of blue dye and isotope with blue dye alone in breast sentinel node biopsy. ANZ J Surg 2005;75:817-21. [PubMed]
- Dabbs DJ, Fung M, Landsittel D, et al. Sentinel lymph node micrometastasis as a predictor of axillary tumor burden. Breast J 2004;10:101-5. [PubMed]
- Olivotto IA, Jackson JSH, Mates D, et al. Prediction of axillary lymph node involvement of women with invasive breast carcinoma. A multivariate analysis. Cancer 1998;83:948-55. [PubMed]
- Joseph KA, El-Tamer M, Komenaka I, et al. Predictors of nonsentinel node metastasis in patients with breast cancer after sentinel node metastasis. Arch Surg 2004;139:648-51. [PubMed]
- Abdessalam SF, Zervos EE, Prasad M, et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg 2001;182:316-20. [PubMed]
- Turner RR, Chu KU, Qi K, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in sentinel lymph node. Cancer 2000;89:574-81. [PubMed]
- Wong SL, Edwards MJ, Chao C, et al. Predicting the status of nonsentinel axillary nodes. Arch Surg 2001;136:563-8. [PubMed]
- Chu KU, Turner RR, Hansen NM, et al. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel node metastasis. Ann Surg Oncol 1999;6:756-61. [PubMed]
- Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol 1999;17:1720-6. [PubMed]
- Kamath VJ, Giuliano R. Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher-echelon nodes in axilla. Arch Surg 2001;136:688-92. [PubMed]


