
Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery
Introduction
The global prevalence of obesity and its associated comorbidities continue to increase on a pandemic scale (1). Recent estimates from the World Health Organization (WHO) indicate that in 2016, over 1.9 billion adults were overweight and, of these, over 650 million were obese (2). Furthermore, 340 million children and adolescents aged 5–19 years and 24 million children under the age of 5 were estimated to be obese or overweight in 2016 (2). Obesity is no longer a public health issue confined to high-income countries, as the developing world is now witnessing increased obesity rates secondary to urbanization, changes in diet, and the adoption of sedentary lifestyles (3). If current trends continue, the global prevalence of obesity is projected to reach 18% in men and exceed 21% in women by 2025 (4). A growing body of evidence supports the notion that obesity is a causative factor in the development of hypertension (5-7). This review provides an overview of the known pathophysiological mechanisms that link excess adiposity with elevated blood pressure (BP) and outlines therapeutic strategies for ameliorating obesity-related hypertension, with a focus on metabolic surgery.
Definitions of obesity and its association with hypertension
Obesity is most accurately defined as the abnormal or excessive accumulation of adiposity to the extent that health may be impaired (2). However, the methods used to directly quantify body fat are cumbersome, expensive, and not routinely available in daily clinical practice (8). For this reason, the body mass index (BMI; body weight in kg divided by height in m2) is the most commonly used surrogate marker of adiposity (9). The WHO defines normal weight as BMI 18.5–24.9 kg/m2; overweight as BMI 25–29.9 kg/m2; and obesity as BMI ≥30 kg/m2 (10). However, BMI does not differentiate between lean muscle and fat mass and does not provide any indication of the distribution of body fat. This is an important consideration as evidence suggests that visceral or retroperitoneal fat (i.e., centrally located body fat) is a more important than peripheral or subcutaneous fat in predicting the risk of cardiometabolic sequalae associated with obesity (11-13). Therefore, alternative anthropometric measures of adiposity such as waist circumference (WC) and waist-to-hip ratio (WHR) have also been utilized (14). Central obesity is defined as a WC of >102 cm in males and >88 cm in females, or a WHR of >1.0 in males and >0.85 in females (15). However, the drawbacks of these indices include the lack of standardized measurement protocols and reference data as well as decreased accuracy in those with severe obesity (BMI >35 kg/m2) (16). Furthermore, the cut-offs for both BMI and WC/WHR were defined based on white European populations, and it is recognized that individuals of Asian descent may have a higher percentage of body fat than individuals of white European descent for a given BMI and WC (17). This has led to the development of ethnicity-specific cut-offs for BMI, WC and WHR in non-white individuals as predictors of cardiometabolic risk (18,19).
The deleterious consequences of obesity include an increased risk of death from cardiovascular disease (CVD) (20), type 2 diabetes mellitus (T2DM) (21), cancer (22), and chronic kidney disease (23). Hypertension, defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg by the European Society of Cardiology/European Society of Hypertension guidelines (24), or systolic BP ≥130 mmHg or diastolic BP ≥80 mmHg in the latest American College of Cardiology (ACC)/American Heart Association (AHA) guidelines (25), is a comorbid condition that is frequently seen in association with obesity (5). Hypertension is currently the leading risk factor for morbidity and mortality worldwide, resulting in 182 million disability-adjusted life years and 10.4 million deaths annually (26). The relationship between obesity and hypertension is well described in children and adults and across both sexes (5,27). For instance, in the Framingham Offspring Study, 78% of new cases of essential hypertension in men and 65% in women were attributable to excess body fat (28). Furthermore, an increase in weight by 5% was associated with a 20–30% increase in the incidence of hypertension (29). In the second Nurses’ Health Study, in which 82,882 adult women were prospectively followed up for 14 years, BMI was the strongest risk factor for developing hypertension, with obese women having almost five times the incidence of hypertension compared to those with BMI <23.0 kg/m2 (30). In concordance with these observations, it has been shown that even modest reductions in weight can decrease BP in hypertensive patients. For example, in the TOHP II (Trials of Hypertension Prevention, phase II) study, in which overweight and obese adults were randomized to a weight loss intervention group versus usual care, participants who maintained a weight reduction of 4.5 kg for 30 months reduced their risk of developing hypertension by 65% (31). The relationship between central obesity measures such as WC/WHR and BP appears to be independent of BMI, and it has been suggested that using these indices in combination, rather than individually, may be a superior predictor of obesity-related cardiometabolic risk in certain populations (32,33).
Mechanisms of obesity-related hypertension
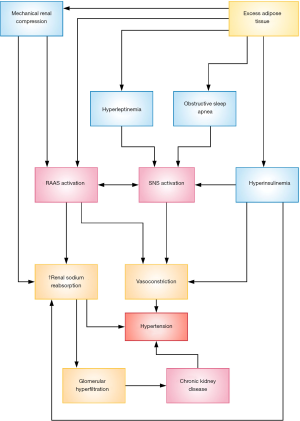
The putative mechanisms underlying obesity-related hypertension are complex and entail interactions between renal, metabolic, and neuroendocrine pathways (Figure 1). These mechanisms include: sympathetic nervous system (SNS) overactivation, stimulation of the renin-angiotensin-aldosterone system (RAAS), alterations in adipose-derived cytokines such as leptin, insulin resistance, and structural as well as functional renal changes.
SNS overactivation
Increased activity of the SNS is believed to play an important role in the development of obesity-related hypertension (34). Physiological manifestations of SNS overactivity include elevations in heart rate, cardiac output, and renal tubular sodium reabsorption; these occur as a direct result of α-adrenergic and β-adrenergic receptor stimulation and indirectly through activation of other systems, such as the RAAS, which is described below. Muscle SNS activity, as measured by microneurography, increases with even modest weight gain (35) and appears to be highest in patients with both obesity and hypertension (36). Renal SNS activity, as measured by the norepinephrine spillover method, is also elevated in obese individuals (37). Notably, the increased SNS activity associated with obesity is not uniformly distributed across all organs, and predominantly affects the kidneys and skeletal muscle (6). On the other hand, the chronic elevation in resting heart rate observed in obese individuals is thought to be mediated by reduced parasympathetic tone rather than increased SNS activity (7). Furthermore, SNS overactivity is not ubiquitously observed in all obese patients, and appears to be influenced by factors such as visceral (rather than subcutaneous) adiposity, ethnicity, and sex (38). For instance, Native American Pima Indians have a high prevalence of obesity, but relatively low rates of hypertension (39,40). One explanation for this may lie in the finding that basal muscle SNS activity is 20–30% lower in Pima Indians compared weight weight-matched white individuals (40). This suggests that SNS activity is a key determinant of obesity-related hypertension and that sympathetic tone in the presence of excess adiposity is influenced by factors such as ethnicity. Further evidence of the association between SNS activity obesity-related hypertension is the finding that pharmacological α/β-adrenergic blockade results in a significantly greater reduction in systolic BP in obese, compared to lean, hypertensive patients (41).
Causative mechanisms of SNS activation in obesity include abnormal adipokine secretion from adipose tissue; stimulation via the RAAS; insulin resistance; and baroreceptor dysfunction (6,7,42). Furthermore, obesity frequently coexists with obstructive sleep apnea (OSA), which results in chronic intermittent hypoxia and leads to the activation of carotid body chemoreceptors that reflexively upregulate SNS activity (43). Some of these mechanisms may also contribute towards the development of hypertension in an SNS-independent manner, which will be discussed below.
Activation of the RAAS
Despite the state of volume expansion and sodium retention associated with obesity, which would normally suppress the RAAS, several reports indicate that obese individuals have higher levels of plasma renin activity, angiotensinogen, angiotensin-converting enzyme (ACE), and aldosterone compared to lean individuals (44,45). Activation of the RAAS leads to increased formation of angiotensin II, which induces systemic vasoconstriction and simulates the production of aldosterone from the adrenal cortex. Both angiotensin II and aldosterone increase renal tubular sodium reabsorption and water retention, resulting in intravascular volume expansion and hypertension.
Several mechanisms are responsible for RAAS activation in obesity. It has been recognized that a bidirectional interaction exists between the SNS and the RAAS, such that the RAAS increases sympathetic tone and, reciprocally, the SNS activates the RAAS (46). This drives the release of renin from the juxtaglomerular cells of the kidney. Renin secretion is also upregulated secondary to physical compression of the kidney by excess visceral and retroperitoneal fat (7). This leads to decreased renal tubular blood flow and sodium delivery, which is sensed by the macula densa, which in turn stimulates renin secretion through tubuloglomerular feedback (47). Adipocytes also possess their own intrinsic RAAS and appear to be major producers of angiotensinogen and angiotensin II (48). Interestingly, mice with adipocyte-specific deficiency of angiotensin are protected from the development of hypertension, despite being fed an obesogenic diet (49). Additionally, adipocytes have been shown to secrete mineralocorticoid-secreting factors that stimulate aldosterone production from the adrenal gland independently of angiotensin II (50,51). The role of RAAS activity in the pathogenesis of obesity-related hypertension in humans is supported by the finding that pharmacologic blockade with ACE inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs) significantly lowers BP in obese patients (52-54).
Functional and structural renal changes
Increased renal sodium reabsorption and volume expansion play an important role in initiating hypertension associated with obesity. As mentioned above, excess visceral and retroperitoneal adiposity can lead to mechanical compression of the kidneys. In addition, the accumulation of peri-renal fat may induce inflammation and expansion of the renal medullary extracellular matrix, which leads to compression of the renal medulla (55). This results in diminished renal tubular blood flow, which prolongs the duration of time in which fractional sodium reabsorption can occur. Consequently, decreased sodium delivery distally to the macula densa stimulates a feedback-mediated reduction in renal afferent arteriolar resistance, an increase in renal blood flow, and stimulation of renin secretion from juxtaglomerular cells (7). These compensatory increases in BP and glomerular filtration rate (GFR) attempt to restore macula densa sodium delivery towards normal levels. Ultimately, however, the elevated glomerular hydrostatic pressure leads to progressive glomerular sclerosis and impaired renal function (56), and a deleterious cycle ensues in which nephrons are injured, sodium retention is exacerbated, and arterial pressures rise to maintain sodium delivery to the macula densa (57). As described earlier, renal SNS activity is upregulated in obesity, and this stimulates sodium reabsorption both directly (58) and indirectly, through activation of the RAAS.
Leptin resistance and hyperleptinemia
Leptin is an adipokine (adipose-derived cytokine) that has a variety of physiological functions, including the regulation of food intake and energy homeostasis (59). In addition, leptin has been shown to simulate SNS activity in the central nervous system and exerts a pressor effect on the cardiovascular system (60). In the normal state, leptin suppresses appetite and increase energy expenditure. However, the observation that obese individuals have high leptin levels in the absence of weight loss, indicates that a state of leptin-resistance exists in obesity (61). This resistance appears to be “selective”, affecting only the appetite-suppressing and metabolic effects of leptin, without attenuating its stimulatory effects on the SNS (62). This has led to the notion that hyperleptinemia, largely through activation of the SNS, may contribute to obesity-related hypertension (63). Although leptin infusions in animal models have been shown to stimulate SNS activity and lead to an increase in BP (64,65), the administration of recombinant leptin for 12 weeks in overweight or obese humans had no effect on BP (66). This illustrates the complexity of the effects of leptin on BP and the need for a better understanding of its role in mediating obesity-related hypertension.
Insulin resistance
Obesity is associated with a state of insulin resistance and hyperinsulinaemia, which may contribute to hypertension through several mechanisms. Insulin is known to exert sympathoexcitatory effects, as evidenced by increased muscle SNS activity following systemic insulin infusion (67,68), and the stimulation of neurons of the paraventricular nucleus (a region of the hypothalamus that is critical to the regulation of sympathetic output) (69). Insulin also directly promotes renal sodium retention in the proximal convoluted tubule via activation of the sodium-hydrogen exchanger 3 (NHE3) (70). Indeed, it has been shown that individuals with metabolic syndrome, of which insulin resistance is a key component, have a significantly higher fractional sodium reabsorption compared to those without metabolic syndrome (71). Insulin is known to act as a vasodilator; however, in obese individuals with chronic hyperinsulinemia this response is blunted secondary to endothelial dysfunction, resulting in a state of increased vasoconstrictor tone (72).
Treatment of obesity-related hypertension
The primary goal of treatment for obesity-related hypertension is weight loss, as this reverses the pathophysiological mechanisms that sustain hypertension. The BP-lowering effects of weight loss appear to be linear, with a decrease in BP of around 1 mmHg reported per kg of weight loss (73), although this effect may be attenuated in the longer-term, with a decrease of around 6 mmHg observed per 10 kg of weight loss (74). Weight reduction is first attempted through nonpharmacological approaches such as lifestyle changes. In patients who are unable to sustain weight loss or recommended BP targets with this approach, adjuvant pharmacotherapy may be required. It is increasingly recognized that metabolic surgery represents an effective strategy for BP control in obese hypertensive patients, and evidence in support of this will be discussed below.
Lifestyle modification
The mainstay of treatment for obesity is lifestyle modification aimed at caloric restriction and increased physical activity (75). Lifestyle interventions consist of dietary adjustments, regular exercise, and behavioural modification. Details regarding specific programs are described elsewhere (5,75) and are beyond the scope of this Review. Unfortunately, rates of recidivism and drop-out are high and few patients succeed in achieving and maintaining long-term weight loss with lifestyle modifications alone (76). This is not solely attributable to a loss of motivation and may also be a result of counter-regulatory hormonal mechanisms that exist to re-establish higher body weight (77).
Pharmacological therapies for obesity
According to guidelines from the ACC/AHA (75) and the Endocrine Society (78), pharmacological therapies for obesity may be considered as useful adjuncts to lifestyle modification for patients with BMI ≥30 kg/m2 or in those with BMI ≥27 kg/m2 and concomitant obesity-related diseases, including hypertension, type 2 diabetes, dyslipidemia and/or OSA. Currently, five drugs are US Food and Drug Administration (FDA)-approved for long-term weight management: orlistat, lorcaserin, phenteramine-topiramate, naltrexone-bupropion, and liraglutide. With the exception of orlistat (which reduces fat absorption by inhibiting gastric and pancreatic lipase), these drugs promote weight loss by reducing food intake and inducing early satiety (79). The degree of weight loss achieved by anti-obesity drugs at over 1 year ranges from approximately 3–9% beyond that achieved with lifestyle modification alone (80). Although a detailed description of each medication is beyond the scope of this Review, it is worth noting that some anti-obesity drugs may have varying effects on BP. For example, orlistat, has been shown to produce placebo-adjusted systolic and diastolic BP reductions of 2.5 and 1.9 mmHg, respectively, in obese hypertensive patients (81). However, it is associated with only a mild degree of weight loss (<3% relative to placebo) (82) and frequent gastrointestinal side effects, such as fecal urgency, incontinence, and oily stool, which hinder its long-term use (79). Compared with placebo, bupropion-naltrexone reduces weight by approximately 4–5% (83), but can raise BP and heart rate and is not recommended for use in hypertensive patients (84). Overall, there is a paucity of data on the efficacy and short- and long-term BP effects of most approved anti-obesity drugs that are specific to obese patients who are also hypertensive (85).
Pharmacological therapies for hypertension in obese individuals
Individuals with obesity are at increased risk of treatment-resistant hypertension (defined as BP that remains above goal despite the concurrent use of three antihypertensive agents of different classes, or controlled BP with >3 medications) (86,87). While most hypertension guidelines do not address obese patients as a distinct population, some recommendations for optimal choice of antihypertensive agent in this group have emerged (5,88). Given the role of the RAAS in the pathogenesis of obesity-related hypertension, there is a strong case for considering ACEIs and ARBs as first-line therapies. These drugs have the added advantage of improving insulin sensitivity (89) and being nephroprotective in patients with diabetes (90), which is a frequent comorbidity in obese individuals. Based on the observation that aldosterone levels are upregulated in obesity, the use of MRAs has also been advocated, although there is little evidence to suggest that these agents show superior efficacy in obese individuals compared with normal weight hypertensive patients (91). Because SNS activation is also implicated in obesity-related hypertension, β-blockers would appear to be a biologically-plausible treatment option. However, many of the drugs in this class are associated with weight gain and insulin resistance, and should be limited to obese patients with specific cardiovascular indications, such as heart failure or post-myocardial infarction (92). When β-blockers are indicated, third generation agents, such as carvedilol and nebivolol, appear to have less weight gain potential and fewer adverse metabolic effects than older β-blockers (93). Calcium channel blockers of the dihydropyridine class have a neutral effect on glucose metabolism and weight gain and are usually recommended as second-line agents in combination with ACEIs/ARBs (88). While thiazide diuretics are useful in counteracting the volume overloaded state that is present in obesity-related hypertension, their metabolic adverse effects include dyslipidemia and insulin resistance, which obese patients treated with these drugs are especially vulnerable to developing (94).
The role of metabolic surgery in the treatment of obesity-related hypertension
Metabolic (also known as bariatric) surgery encompasses some of the fastest-growing gastrointestinal procedures worldwide. According to the latest International Federation for the Surgery of Obesity and Metabolic Disorders survey, an estimated >680,000 metabolic procedures were performed across the globe in 2016 (95), which was double the number of cases reported in 2011 (96). Metabolic procedures currently performed in the US (in decreasing order of frequency) include sleeve gastrectomy (SG, 58%); Roux-en-Y gastric bypass (RYGB, 19%); adjustable gastric banding (AGB, 3%); and biliopancreatic diversion with duodenal switch (BPD/DS, 0.6%) (97).
Metabolic surgery has emerged as the most successful strategy for achieving substantial and durable weight loss in obese individuals, as shown in the pooled analyses of several randomized control trials (RCTs) and observational studies (98-102). For example, in a meta-analysis of 11 RCTs containing a total of 796 obese individuals, those who underwent metabolic surgery experienced 26 kg greater weight loss compared to those who were subjected to non-surgical treatment (101). With regards to surgery type, BPD/DS provides the most significant weight loss out of all bariatric procedures, because it involves the most extensive degree of intestinal bypass (103). However, it is also the most technically challenging, and is therefore the least frequently performed (97). RYGB was formerly the most common bariatric procedure, but has now largely been supplanted by SG, as the latter is considered to be a more straightforward and quicker operation, with a lower complication rate (104). While some studies have shown superior weight loss outcomes with RYGB (105,106), which is considered by many to be the “gold standard” weight loss procedure, others have demonstrated similar efficacy between RYGB and SG (107,108). There is, however, consensus that both operations result in significantly greater and longer-lasting weight loss than AGB, a procedure that is now rarely performed in contemporary bariatric practice (109).
In addition to substantial effects on weight loss, there is now an impressive array of evidence to show that metabolic surgery is effective in treating T2DM (110-115). Indeed, many patients who undergo these procedures achieve complete remission of T2DM, defined by the American Diabetes Association (ADA) as a glycated haemoglobin (HBA1c) <6.0% or fasting glucose <100 mg/dL of at least 1 year’s duration in the absence of pharmacological therapies (116). For instance, in the STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) trial, 150 patients with T2DM and BMI 27 to 43 kg/m2 were randomized to RYGB, SG, or intensive medical therapy alone (117). At 1 year, 12% of patients in the medical therapy group achieved the trial’s primary outcome of HbA1c <6.0%, compared to 42% of patients who underwent RYGB (P=0.002) and 37% who underwent SG (P=0.003). Follow-up data from this trial showed that the beneficial effects of metabolic surgery on glycemic control were durable and remained significantly greater than medical therapy alone at 5 years (112). The widespread success of metabolic surgery is reflected in its inclusion within treatment algorithms for T2DM proposed by international diabetes organisations (118).
Although the majority of studies have focused on the beneficial effects of metabolic surgery in terms of BMI and glycemic control, there is growing interest in exploring the impact of these procedures on obesity-associated CVDs. Indeed, meta-analyses of mostly observational studies have suggested that metabolic surgery confers a beneficial effect in patients with hypertension (98,119-124). In an extensive systematic review by Vest et al. (122), which included 73 studies and 19,543 individuals undergoing a range of bariatric procedures including SG, RYGB, ABG, and SG, postoperative resolution or improvement of hypertension occurred in 63% of patients, with follow-up ranging from 3 months to ~15 years. These results were corroborated in a separate meta-analysis of 57 studies containing 51,241 patients by Wilhelm et al. (124), which showed improvement in hypertension in 63.7% of patients who underwent metabolic surgery and had follow-up ranging from 1 week to 7 years. In a subgroup analysis of studies that reported resolution of hypertension, the authors noted that this outcome occurred in 50% of patients. However, a long-term (>5 years) beneficial effect on BP has not invariably been observed in all studies. For example, in the Swedish Obese Subjects (SOS) study, in which 2,010 obese individuals were prospectively followed up after metabolic surgery and compared with 2,037 contemporaneously-matched controls (125), no significant difference in the incidence of hypertension was observed between the two groups at 2 years (34% surgery vs. 21% control) and 10 years (19% surgery vs. 10% control). However, limitations of the SOS study include its nonrandomized design and the fact that BP was not the primary outcome. Furthermore, as the study was initiated over 25 years ago, most patients underwent vertical-banded gastroplasty (an obsolete procedure that is now rarely performed) and only 34 of the patients with 10 years of follow-up data underwent RYGB. In a subsequent analysis of SOS data, which included a larger number of RYGB patients with long-term follow-up (126), patients in the surgery group actually had a significant reduction in systolic/diastolic BP by 12.1/7.3 and 5.1/5.6 mmHg at 2 and 10 years of follow-up, respectively. In a separate prospective cohort study of 197 patients who underwent RYGB or SG, relapse of hypertension at 3 years was also observed in >20% of patients who achieved apparent hypertension remission at 12 months (127). The only independent predictor of relapse was the use of a greater number of preoperative antihypertensive medication. This suggests a waning effect of metabolic surgery on BP control over time, although further high-quality data regarding the mid- and long-term effects on hypertension remission are required.
It is important to note that that the majority of observational studies and all of the RCTs mentioned above have focused on weight loss or T2DM resolution as primary end-points. To date, only one RCT has set out to evaluate the impact of metabolic surgery on hypertension as a primary outcome in obese patients (128). In the GATEWAY (Gastric Bypass to Treat Obese Patients with Steady Hypertension) trial, 100 patients with obesity and hypertension were randomized 1:1 to undergo RYGB plus medical therapy or medical therapy alone (128). Patients with known CVD or poorly controlled T2DM were excluded. At 1 year, the primary outcome (a reduction in the total number of antihypertensive medications by ≥30%) was 6 times more likely in patients who underwent surgery (84% vs. 13%). Furthermore, remission of hypertension (defined as a BP <140/90 mmHg without medication use) was attained in significantly more patients in the surgery group (56% vs. 0%). In a subsequently-published sub-study by the GATEWAY trial authors (129), patients who underwent RYGB were found to have significantly less BP variability at 1 year and a lower prevalence of resistant hypertension compared to those who received medical therapy alone (10% vs. 15%). This is a noteworthy finding as BP variability has been shown to increase the risk of adverse cardiovascular events (130), and correlates with the severity of target end-organ damage in hypertensive individuals (131). An important limitation of the GATEWAY trial is the limited duration of follow-up, although the investigators intend to report 5-year outcomes in the future (132). Furthermore, the single-centre nature of the study and exclusion of patients with BMI 30–39.9 kg/m2 may limit the generalizability of the findings. Finally, all the patients in the surgery group underwent RYGB, despite the fact that SG is now the most-commonly performed metabolic procedure worldwide. Nonetheless, the GATEWAY trial provides the first Level I evidence that metabolic surgery is an effective treatment for obesity-related hypertension and its findings are in concordance with the majority of prior observational studies.
Conclusions and future perspectives
Obesity is one of the greatest public health challenges in modern times, and is inextricably linked to adverse cardiovascular risk. Obesity-related hypertension occurs due to the complex interplay between multiple mechanisms including inappropriate activation of the SNS and RAAS, adipocyte dysfunction, and impaired pressure natriuresis that is exacerbated by physical compression of the kidneys. Weight loss is the cornerstone of treatment for obesity and its metabolic consequences. However, many patients are unable to achieve and sustain an adequate degree of weight loss through lifestyle measures alone. While a number of anti-obesity medications exist, concerns regarding their safety and efficacy in hypertensive patients remain. Patients with obesity are also more likely to have hypertension that is resistant to treatment with multiple anti-hypertensive agents. Metabolic surgery is currently the most effective treatment for achieving durable weight loss and has been shown to have beneficial effects in patients with T2DM. It is increasingly recognized that the benefits of metabolic surgery also extend to hypertension remission; however, little is known about the causal mechanisms underlying this success. Because a reduction in BP may occur as early as 1 week postoperatively (133) (i.e., before the onset of adipose mass loss) it is plausible that neurohormonal mechanisms are implicated in the BP-lowering effects of bariatric surgery. Further studies, including research in pre-clinical models, are needed to better understand these mechanisms. While the accumulating evidence base is promising, further high-quality data regarding long-term BP outcomes following both RYGB and SG are needed to confirm that the beneficial effects on hypertension are durable. Although metabolic surgery, with its potential risks, should not be considered a first-line treatment for isolated hypertension, it warrants consideration as a viable option for the subset of obese patients with hypertension that is severe or refractory to alternative treatments. As the global burden and severity of obesity and hypertension continue to increase, research that examines the impact of metabolic surgery on ameliorating these conditions will remain of crucial importance for the years to come.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Afshin A, Forouzanfar MH, Reitsma MB, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377:13-27. [Crossref] [PubMed]
- Organization WH. Obesity and overweight. 2018. Available online: . Accessed September 1 2019.https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- Fox A, Feng W, Asal V. What is driving global obesity trends? Globalization or "modernization"? Global Health 2019;15:32. [Crossref] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377-96. [Crossref] [PubMed]
- Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14-33. [Crossref] [PubMed]
- Hall JE, da Silva AA, do Carmo JM, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010;285:17271-6. [Crossref] [PubMed]
- Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015;116:991-1006. [Crossref] [PubMed]
- Gurunathan U, Myles PS. Limitations of body mass index as an obesity measure of perioperative risk. Br J Anaesth 2016;116:319-21. [Crossref] [PubMed]
- Gallagher D, Visser M, Sepulveda D, et al. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996;143:228-39. [Crossref] [PubMed]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 1998;6 Suppl 2:51S-209S. [PubMed]
- Onat A, Avci GS, Barlan MM, et al. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord 2004;28:1018-25. [Crossref] [PubMed]
- Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301-13. [Crossref] [PubMed]
- Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961-9. [Crossref] [PubMed]
- Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ 2018;360:k1274. [Crossref] [PubMed]
- World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. 2011.
- Wang J, Gallagher D, Thornton JC, et al. Regional body volumes, BMI, waist circumference, and percentage fat in severely obese adults. Obesity (Silver Spring) 2007;15:2688-98. [Crossref] [PubMed]
- Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. [Crossref] [PubMed]
- Misra A, Vikram NK, Gupta R, et al. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int J Obes (Lond) 2006;30:106-11. [Crossref] [PubMed]
- Katzmarzyk PT, Bray GA, Greenway FL, et al. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring) 2011;19:1272-8. [Crossref] [PubMed]
- Hubert HB, Feinleib M, McNamara PM, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67:968-77. [Crossref] [PubMed]
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors, 2001. JAMA 2003;289:76-9. [Crossref] [PubMed]
- Calle EE, Thun MJ. Obesity and cancer. Oncogene 2004;23:6365-78. [Crossref] [PubMed]
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260-72. [Crossref] [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127-248. [Crossref] [PubMed]
- GBD 2017 RIsk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923-94. [Crossref] [PubMed]
- Faulkner JL, Belin de Chantemele EJ. Sex Differences in Mechanisms of Hypertension Associated With Obesity. Hypertension 2018;71:15-21. [Crossref] [PubMed]
- Garrison RJ, Kannel WB, Stokes J 3rd, et al. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 1987;16:235-51. [Crossref] [PubMed]
- Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet 2001;358:1682-6. [Crossref] [PubMed]
- Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 2009;302:401-11. [Crossref] [PubMed]
- Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001;134:1-11. [Crossref] [PubMed]
- Zhang M, Zhao Y, Wang G, et al. Body mass index and waist circumference combined predicts obesity-related hypertension better than either alone in a rural Chinese population. Sci Rep 2016;6:31935. [Crossref] [PubMed]
- Luz RH, Barbosa AR, d'Orsi E. Waist circumference, body mass index and waist-height ratio: Are two indices better than one for identifying hypertension risk in older adults? Prev Med 2016;93:76-81. [Crossref] [PubMed]
- Lambert EA, Esler MD, Schlaich MP, et al. Obesity-Associated Organ Damage and Sympathetic Nervous Activity. Hypertension 2019;73:1150-9. [Crossref] [PubMed]
- Gentile CL, Orr JS, Davy BM, et al. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol 2007;292:R1834-8. [Crossref] [PubMed]
- Lambert E, Straznicky N, Schlaich M, et al. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 2007;50:862-8. [Crossref] [PubMed]
- Vaz M, Jennings G, Turner A, et al. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 1997;96:3423-9. [Crossref] [PubMed]
- Alvarez GE, Ballard TP, Beske SD, et al. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol 2004;287:H414-8. [Crossref] [PubMed]
- Saad MF, Lillioja S, Nyomba BL, et al. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med 1991;324:733-9. [Crossref] [PubMed]
- Weyer C, Pratley RE, Snitker S, et al. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension 2000;36:531-7. [Crossref] [PubMed]
- Wofford MR, Anderson DC Jr, Brown CA, et al. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens 2001;14:694-8. [Crossref] [PubMed]
- Grassi G, Dell'Oro R, Facchini A, et al. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens 2004;22:2363-9. [Crossref] [PubMed]
- Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266-74. [Crossref] [PubMed]
- Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab 2007;92:4472-5. [Crossref] [PubMed]
- Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 2001;79:21-9. [Crossref] [PubMed]
- Cabandugama PK, Gardner MJ, Sowers JR. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med Clin North Am 2017;101:129-37. [Crossref] [PubMed]
- Hall ME, do Carmo JM, da Silva AA, et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014;7:75-88. [Crossref] [PubMed]
- Schütten MT, Houben AJ, de Leeuw PW, et al. The Link Between Adipose Tissue Renin-Angiotensin-Aldosterone System Signaling and Obesity-Associated Hypertension. Physiology (Bethesda) 2017;32:197-209. [Crossref] [PubMed]
- Yiannikouris F, Karounos M, Charnigo R, et al. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol 2012;302:R244-51. [Crossref] [PubMed]
- Jeon JH, Kim KY, Kim JH, et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 2008;22:1502-11. [Crossref] [PubMed]
- Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A 2003;100:14211-6. [Crossref] [PubMed]
- Grassi G, Seravalle G, Dell'Oro R, et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens 2003;21:1761-9. [Crossref] [PubMed]
- Dorresteijn JA, Schrover IM, Visseren FL, et al. Differential effects of renin-angiotensin-aldosterone system inhibition, sympathoinhibition and diuretic therapy on endothelial function and blood pressure in obesity-related hypertension: a double-blind, placebo-controlled cross-over trial. J Hypertens 2013;31:393-403. [Crossref] [PubMed]
- Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059-68. [Crossref] [PubMed]
- Dwyer TM, Banks SA, Alonso-Galicia M, et al. Distribution of renal medullary hyaluronan in lean and obese rabbits. Kidney Int 2000;58:721-9. [Crossref] [PubMed]
- Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498-509. [Crossref] [PubMed]
- Kotsis V, Stabouli S, Papakatsika S, et al. Mechanisms of obesity-induced hypertension. Hypertens Res 2010;33:386-93. [Crossref] [PubMed]
- Healy V, Thompson C, Johns EJ. The adrenergic regulation of proximal tubular Na(+)/H(+) exchanger 3 in the rat. Acta Physiol (Oxf) 2014;210:678-89. [Crossref] [PubMed]
- Schwartz MW, Woods SC, Porte D Jr, et al. Central nervous system control of food intake. Nature 2000;404:661-71. [Crossref] [PubMed]
- Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 1998;31:409-14. [Crossref] [PubMed]
- Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292-5. [Crossref] [PubMed]
- Rahmouni K, Morgan DA, Morgan GM, et al. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 2005;54:2012-8. [Crossref] [PubMed]
- Kshatriya S, Liu K, Salah A, et al. Obesity hypertension: the regulatory role of leptin. Int J Hypertens 2011;2011:270624. [Crossref] [PubMed]
- Haynes WG, Morgan DA, Walsh SA, et al. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 1997;100:270-8. [Crossref] [PubMed]
- Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 1997;46:2040-3. [Crossref] [PubMed]
- Zelissen PM, Stenlof K, Lean ME, et al. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab 2005;7:755-61. [Crossref] [PubMed]
- Anderson EA, Balon TW, Hoffman RP, et al. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 1992;19:621-7. [Crossref] [PubMed]
- Gudbjörnsdottir S, Elam M, Sellgren J, et al. Insulin increases forearm vascular resistance in obese, insulin-resistant hypertensives. J Hypertens 1996;14:91-7. [PubMed]
- Ward KR, Bardgett JF, Wolfgang L, et al. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 2011;57:435-41. [Crossref] [PubMed]
- DeFronzo RA, Cooke CR, Andres R, et al. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975;55:845-55. [Crossref] [PubMed]
- Strazzullo P, Barbato A, Galletti F, et al. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J Hypertens 2006;24:1633-9. [Crossref] [PubMed]
- Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 2013;14:5-12. [Crossref] [PubMed]
- Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003;42:878-84. [Crossref] [PubMed]
- Aucott L, Poobalan A, Smith WC, et al. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension 2005;45:1035-41. [Crossref] [PubMed]
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129:S102-38. [Crossref] [PubMed]
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond) 2015;39:1188-96. [Crossref] [PubMed]
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597-604. [Crossref] [PubMed]
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:342-62. [Crossref] [PubMed]
- Saunders KH, Umashanker D, Igel LI, et al. Obesity Pharmacotherapy. Med Clin North Am 2018;102:135-48. [Crossref] [PubMed]
- Gadde KM, Pritham Raj Y. Pharmacotherapy of Obesity: Clinical Trials to Clinical Practice. Curr Diab Rep 2017;17:34. [Crossref] [PubMed]
- Siebenhofer A, Jeitler K, Horvath K, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev 2016;3:CD007654. [PubMed]
- Sahebkar A, Simental-Mendia LE, Kovanen PT, et al. Effects of orlistat on blood pressure: a systematic review and meta-analysis of 27 randomized controlled clinical trials. J Am Soc Hypertens 2018;12:80-96. [Crossref] [PubMed]
- Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab 2009;94:4898-906. [Crossref] [PubMed]
- Nissen SE, Wolski KE, Prcela L, et al. Effect of Naltrexone-Bupropion on Major Adverse Cardiovascular Events in Overweight and Obese Patients With Cardiovascular Risk Factors: A Randomized Clinical Trial. JAMA 2016;315:990-1004. [Crossref] [PubMed]
- Cohen JB, Gadde KM. Weight Loss Medications in the Treatment of Obesity and Hypertension. Curr Hypertens Rep 2019;21:16. [Crossref] [PubMed]
- Booth HP, Prevost AT, Gulliford MC. Severity of obesity and management of hypertension, hypercholesterolaemia and smoking in primary care: population-based cohort study. J Hum Hypertens 2016;30:40-5. [Crossref] [PubMed]
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008;117:e510-26. [Crossref] [PubMed]
- Allcock DM, Sowers JR. Best strategies for hypertension management in type 2 diabetes and obesity. Curr Diab Rep 2010;10:139-44. [Crossref] [PubMed]
- Koh KK, Quon MJ, Han SH, et al. Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol 2010;140:73-81. [Crossref] [PubMed]
- Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329:1456-62. [Crossref] [PubMed]
- Byrd JB, Brook RD. A critical review of the evidence supporting aldosterone in the etiology and its blockade in the treatment of obesity-associated hypertension. J Hum Hypertens 2014;28:3-9. [Crossref] [PubMed]
- Lee P, Kengne AP, Greenfield JR, et al. Metabolic sequelae of beta-blocker therapy: weighing in on the obesity epidemic? Int J Obes (Lond) 2011;35:1395-403. [Crossref] [PubMed]
- Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertens (Greenwich) 2009;11:309-15. [Crossref] [PubMed]
- Cooper-DeHoff RM, Wen S, Beitelshees AL, et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension 2010;55:61-8. [Crossref] [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg 2018;28:3783-94. [Crossref] [PubMed]
- Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013;23:427-36. [Crossref] [PubMed]
- English WJ, DeMaria EJ, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis 2018;14:259-63. [Crossref] [PubMed]
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]
- Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 2014;149:275-87. [Crossref] [PubMed]
- Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014.CD003641. [PubMed]
- Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934. [Crossref] [PubMed]
- Puzziferri N, Roshek TB 3rd, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA 2014;312:934-42. [Crossref] [PubMed]
- Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg 2012;147:847-54. [Crossref] [PubMed]
- Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 2013;258:690-4; discussion 695. [Crossref] [PubMed]
- Ignat M, Vix M, Imad I, et al. Randomized trial of Roux-en-Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg 2017;104:248-56. [Crossref] [PubMed]
- Osland E, Yunus RM, Khan S, et al. Weight Loss Outcomes in Laparoscopic Vertical Sleeve Gastrectomy (LVSG) Versus Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) Procedures: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Surg Laparosc Endosc Percutan Tech 2017;27:8-18. [PubMed]
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 2018;319:255-65. [Crossref] [PubMed]
- Kang JH, Le QA. Effectiveness of bariatric surgical procedures: A systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e8632. [Crossref] [PubMed]
- Piché ME, Auclair A, Harvey J, et al. How to choose and use bariatric surgery in 2015. Can J Cardiol 2015;31:153-66. [Crossref] [PubMed]
- Yu J, Zhou X, Li L, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg 2015;25:143-58. [Crossref] [PubMed]
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577-85. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med 2017;376:641-51. [Crossref] [PubMed]
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240-9. [Crossref] [PubMed]
- Liang Z, Wu Q, Chen B, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50-6. [Crossref] [PubMed]
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964-73. [Crossref] [PubMed]
- Buse JB, Caprio S, Cefalu WT, et al. How Do We Define Cure of Diabetes? Diabetes Care 2009;32:2133-5. [Crossref] [PubMed]
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-76. [Crossref] [PubMed]
- Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016;39:861-77. [Crossref] [PubMed]
- Ricci C, Gaeta M, Rausa E, et al. Early Impact of Bariatric Surgery on Type II Diabetes, Hypertension, and Hyperlipidemia: A Systematic Review, Meta-Analysis and Meta-Regression on 6,587 Patients. Obes Surg 2014;24:522-8. [Crossref] [PubMed]
- Ricci C, Gaeta M, Rausa E, et al. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg 2015;25:397-405. [Crossref] [PubMed]
- Heneghan HM, Meron-Eldar S, Brethauer SA, et al. Effect of Bariatric Surgery on Cardiovascular Risk Profile†Drs. Heneghan and Meron-Eldar contributed equally to this article. Am J Cardiol 2011;108:1499-507. [Crossref] [PubMed]
- Vest AR, Heneghan HM, Agarwal S, et al. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012;98:1763-77. [Crossref] [PubMed]
- Sarkhosh K, Birch DW, Shi X, et al. The impact of sleeve gastrectomy on hypertension: a systematic review. Obes Surg 2012;22:832-7. [Crossref] [PubMed]
- Wilhelm SM, Young J, Kale-Pradhan PB. Effect of bariatric surgery on hypertension: a meta-analysis. Ann Pharmacother 2014;48:674-82. [Crossref] [PubMed]
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [Crossref] [PubMed]
- Hallersund P, Sjöström L, Olbers T, et al. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis - long term results from the Swedish Obese Subjects (SOS) study. PLoS One 2012;7:e49696. [Crossref] [PubMed]
- Benaiges D, Sague M, Flores-Le Roux JA, et al. Predictors of Hypertension Remission and Recurrence After Bariatric Surgery. Am J Hypertens 2016;29:653-9. [Crossref] [PubMed]
- Schiavon CA, Bersch-Ferreira AC, Santucci EV, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation 2018;137:1132-42. [Crossref] [PubMed]
- Schiavon CA, Ikeoka D, Santucci EV, et al. Effects of Bariatric Surgery Versus Medical Therapy on the 24-Hour Ambulatory Blood Pressure and the Prevalence of Resistant Hypertension: The GATEWAY Randomized Clinical Trial. Hypertension 2019;73:571-7. [Crossref] [PubMed]
- Pringle E, Phillips C, Thijs L, et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens 2003;21:2251-7. [Crossref] [PubMed]
- Parati G, Pomidossi G, Albini F, et al. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 1987;5:93-8. [Crossref] [PubMed]
- Schiavon CA, Ikeoka DT, de Sousa MG, et al. Effects of gastric bypass surgery in patients with hypertension: rationale and design for a randomised controlled trial (GATEWAY study). BMJ Open 2014;4:e005702. [Crossref] [PubMed]
- Ahmed AR, Rickards G, Coniglio D, et al. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg 2009;19:845-9. [Crossref] [PubMed]


