
The role of multimodal navigation in endoscopic endonasal surgery for giant pituitary adenomas
Introduction
Giant pituitary adenomas (GPAs), which are defined as tumors with a maximum diameter larger than 40 mm, account for 5–14% of adenomas that are treated surgically (1,2). Surgery for patients with a GPA is challenging due to the enormous tumor size, irregular extension, and invasiveness. Gross total resection (GTR) is achieved in less than 50% of GPAs, with a reported 10% to 20% complication rate (1,3,4). Surgery remains the main treatment option for GPA, excluding most prolactinomas. Recently, with advances in endoscopic surgical techniques, a purely endoscopic endonasal surgery (EES) has gained acceptance for the surgical treatment of GPA (5-7). Additionally, in comparisons with microsurgical approaches, EES has shown equivalent or superior outcomes with respect to length of stay, rate of resection, postoperative diabetes insipidus (DI), incidence of cerebrospinal fluid (CSF) leaks, and visual outcomes (4,8-12). The most advantageous features of adapting EES are the panoramic views enabled through the angled endoscopes which generally allow visualization of lesion boundaries, neurovascular structures, and its suprasellar extension (13-15). However, for large and giant adenomas, there is a risk of vascular injury or cranial nerve damage from resecting tumor grossly invading the cavernous sinus (8,10,16,17). Additionally, inadequate tumor resection occasionally causes subdural and subarachnoid hemorrhage and peritumoral swelling because of hemorrhage from the residual tumor and results in deteriorated visual and neurologic outcomes (6,18,19). The primary goal of GPA is complete resection of the tumor with maximal preservation of the normal pituitary gland.
Advance in EES for treatment of pituitary tumors has involved the implementation of intraoperative navigation systems and micro-Doppler, which enable enhanced visualization of adjacent vascular, neural, and ventricular structures (20). Image-based pre-operative vascular and neural element segmentation is highly informative preoperatively and could help young and inexperienced neurosurgeons to avoid vascular and neural injury during trans-sphenoidal surgeries, as well as provide reassurance to more experienced surgeons (20). The effectiveness of multimodal navigation has been proven and is occasionally used in EES; however, whether the implementation of intraoperative multimodal navigation in EES for GPA would improve the removal rates and clinical outcomes has not been substantially addressed.
The purpose of this study was to review the clinical outcomes of a series of 60 consecutive patients with GPA who had undergone EES and to investigate the efficacy and complications with respect to the application of intraoperative multimodal navigation.
Methods
Patient population and study design
After obtaining approval from the review board of the Tangdu Hospital Institutional (No. TDLL-2011034), we retrospectively reviewed a database of more than 300 consecutive cases of endoscopic endonasal pituitary adenoma recorded between January 2012 and December 2015. All surgeries were primarily performed by the lead neurosurgeon (Dong Jia) during this time period. All clinical data, including medical records, radiologic evaluations, laboratory data, and pathologic examinations, were retrospectively reviewed until March 2019. Inclusion criteria were patients with pathology confirmed pituitary adenoma with a maximum tumor diameter >4 cm in at least 1 dimension and an estimated tumor volume >10 cm3. Patients who did not complete the follow-up nor had no postoperative MRI were excluded from the study. Based on whether intraoperative multimodal navigation was applied, 60 GPA patients were categorized into two groups: the standard group and the navigation group. Given the study design of a retrospective chart review, patient consent was not required.
Surgical techniques
All procedures were performed using a purely endoscopic endonasal approach, which was performed with a 2-surgeon, 3-hand technique via a single nostril. The endoscope was manually managed by an assistant. Under general anesthesia, the patient was placed supine with the head in a neutral position, and the trunk elevated around 30°. For the cases in the navigation group, the patient’s head was registered in the intra-op neuronavigation system (Stealth Station; Medtronic, TN, USA). After surgical exposure, each segmented neural or vascular element was validated by manual placement of the navigation probe directly on that object or as closely as possible to the target. A vascularized nasoseptal flap based on the sphenopalatine artery was prepared for reconstruction in almost all cases, as described previously (21). The middle turbinate was preserved routinely. The anterior portion of the vomer bone, rostrum of the sphenoid bone, and the bony septum inside the sphenoid sinus were removed by high-speed drilling. The extent of the bony removal of sellar floor depended on the shape of the lesion. In cases with notable suprasellar extension, an extended transsphenoidal approach was used so that the bone over the planum and/or tuberculum would be removed. The navigation system was used to determine boundaries between the lesion and normal and eloquent structures before the bony removal in the navigation group. Meanwhile, in the standard group, the scope of bony removal depended on preoperative imaging and the surgeon’s experiences. In cases with significant cavernous sinus invasion, the sellar floor overlying the carotid protuberance was removed. In the navigation group, a micro-Doppler was used to detect the audible pulses of the bilateral internal carotid artery (ICA) to identify the trajectory of the carotid artery prior to dural opening, particularly for exposure of the cavernous sinus. After the dural opening, the resection of the tumor was performed in an extracapsular fashion whenever feasible. Lesionectomy was performed using microscissors, ring curettes, suctions, and an ultrasonic aspirator if necessary. The navigation system was used to confirm the extent of lesion resection and minimize residual tumors before reconstruction. Skull base reconstruction was performed in a multilayer fashion using artificial dura mater, SURGICEL (Ethicon Inc.), tissue glue, gelfoam, and the vascularized nasoseptal flap.
Data collection
All pre- and postoperative radiologic evaluations, endocrinology studies, postoperative outcomes, complications, and clinical records were compared for analysis. Visual acuity and Humphrey visual field tests were performed by an independent neuro-ophthalmologist for all patients preoperatively and approximately 12 weeks postoperatively. The records of visual deficits, laterality of deficits, and duration of deficits before surgery were analyzed.
Laboratory examinations were performed preoperatively and approximately 3 months postoperatively in all patients and included tests for triiodothyronine (T3), thyroxine (T4), free T4, thyrotropin-stimulating hormone, adrenocorticotropic hormone, cortisol, serum growth hormone, insulin-like growth factor 1, prolactin, follicle-stimulating hormone, luteinizing hormone, and either testosterone in males or estrogen in females. Postoperatively, all patients were routinely monitored for serum sodium, urine output, and particular attention was paid to DI and electrolyte imbalance.
All patients underwent preoperative magnetic resonance imaging (MRI) and computed tomography scan for evaluation of the size, location, and extension of the pituitary mass lesion. The parameters including the maximum tumor diameters, lobulated tumor configuration, and the intracranial extension index (defined as the approximate ratio of intracranial to total tumor volume measured by sagittal and coronal images) were assessed on preoperative MRI. All patients underwent postoperative MRI within 72 hours or at approximately 12 weeks postoperatively. The extent of tumor resection was evaluated based on the first postoperative MRI and categorized into 3 statuses, which were GTR, near-total resection (NTR) (>90%), or subtotal resection (STR) (<90%), according to the completeness of resection. When GTR was not achieved, the volume of residual tumor was calculated using the following formula: (A×B×C)/2, where A, B, and C are the maximum tumor diameters in each of the 3 dimensions.
Complications included CSF leak, visual disturbance, hematoma, new pituitary deficit, DI, and other clinical deterioration during the first 30 days postoperatively that needed further management.
Statistical analysis
Preoperative patient characteristics, clinical outcomes, and extent of resection, and complications of the two groups were statistically compared. The data were analyzed using version 17.0 of SPSS (SPSS, Chicago, IL, USA) software. Variables were classified as continuous or categorical. Independent Student’s t-tests were used for continuous variables between the two groups. Fisher’s exact test or Pearson’s χ2 test was used for comparison of categorical variables among groups. Factors associated with tumor GTR were analyzed by multinomial logistic regression analysis. A two-tailed P value <0.05 was considered statistically significant.
Results
Patient demographics and clinical presentation
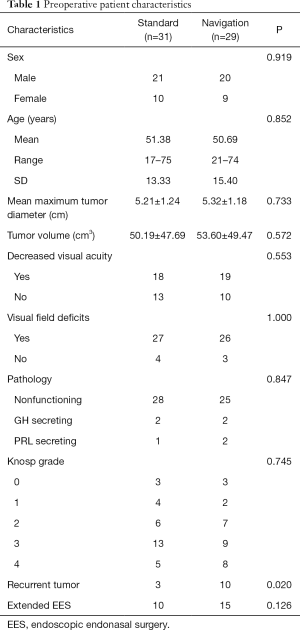
A total of 60 consecutive patients who received EES for GPA were included in the study, which accounted for 19.87% of 302 cases of pituitary adenomas who received surgery during the study period. Patients were classed into two groups: the standard group (n=31) and the navigation group (n=29). There was no significant difference between the two groups in terms of patient sex, age, mean maximum tumor diameter, tumor volume, visual dysfunction, tumor pathology, Knosp grade, and the use of extended EES (Table 1). However, there were 10 cases of recurrent tumors after previous endoscopic or microscopic transsphenoidal surgery in the navigation group which was significantly more than those in the standard group (P=0.02). The mean age at the time of EES was 51.38±13.33 years (mean ± SD) in the standard group and 50.69±15.40 years in the navigation group, both with a male predominance (67.74% and 68.96%, respectively). GPAs were confirmed by both preoperative MRI and postoperative histopathologic diagnosis, for tumor size and pathology, respectively. The mean maximum tumor diameter in the preoperative MRI was 5.21±1.24 cm in the standard group and 5.32±1.18 cm in the navigation group, while the preoperative mean tumor volume was 50.19±47.69 and 53.60±49.47 cm3, respectively. The most common presenting symptom was visual field deficits (87.09% in the standard group and 89.65% in the navigation group) and decreased visual acuity (58.06% and 65.51%, respectively). The most common pathology of tumor was nonfunctional adenomas (28 in the standard group and 25 in the navigation group). The functional GPAs included 4 growth hormone-secreting tumors and 3 medically resistant prolactinomas. Based on the Knosp classification, there were 3, 4, 6, 13, and 5 patients of Knosp grade 0, 1, 2, 3, and 4 in the standard group, and 3, 2, 7, 9, and 8 in the navigation group, respectively (Table 1).
Full table
The extent of resection and clinical outcomes
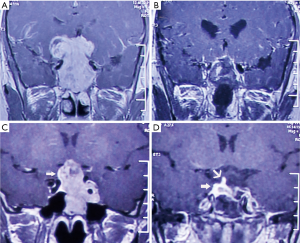
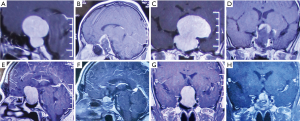
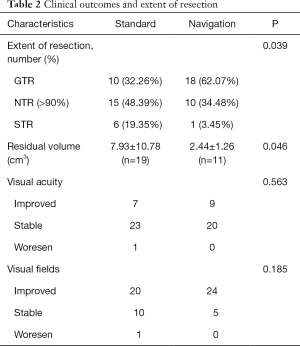
The overall mean follow-up time was 42.53±10.29 months. There was a significant difference between the two groups in the extent of resection (P=0.039). GTR was achieved in 10 patients (32.26%) in the standard group, which was significantly lower than that in the navigation group (18/29=62.07%) (Figure 1). NTR was achieved in 15 patients (48.39%) in the standard group and 10 patients (34.48%) in the navigation group (Figure 2), while STR was achieved in 6 patients (19.35%) and in 1 patient (3.45%), respectively (Table 2). For the residual tumors, the mean residual volume was compared between the two groups. The residual volume was 7.93±10.78 cm3 in the standard group which was significantly more than that in the navigation group (2.44±1.26 cm3, P=0.046).
Full table
All patients completed postoperative visual acuity and visual field tests. Although the rates of visual acuity and visual field improvement were much higher in the navigation group, there was no significant difference between the two groups (Table 2). For the visual acuity, 18 patients in the standard group and 19 patients in the navigation group decreased preoperatively, and of these, 7 (7/18=38.89%) patients and 9 (9/19=47.37%) patients had visual acuity improvement, while 23 patients and 20 patients had no changes, respectively. Only 1 patient in the standard group with decreased preoperative visual acuity and visual field deficit developed visual deterioration after surgery. For the visual field, 27 patients in the standard group and 26 patients in the navigation group had preoperative visual field deficits, and of these, 20 (20/27=74.07%) patients and 24 (24/26=92.31%) patients had visual field improvement, while 10 patients and 5 patients had no changes, respectively (Table 2).
Complications
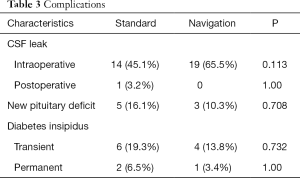
CSF leak was the most common complication of EES, especially during operation. There were 14 patients (45.1%) in the standard group and 19 patients (65.5%) in the navigation group in whom intraoperative CSF leak occurred. However, there was only 1 patient who developed a postoperative CSF leak in the standard group and no patients in the navigation group who had postoperative CSF leak. There was no significant difference between the two groups regarding CSF leak (Table 3). The intraoperative CSF leak was managed immediately in a multilayer fashion after tumor resection. All but 1 intraoperative CSF leak patient in the standard group was successfully repaired, and there was no postoperative CSF rhinorrhea or related meningitis. The patient had a postoperative CSF leak, which was successfully treated with 7 days’ lumbar drainage.
Full table
There were 5 patients (16.1%) in the standard group and 3 patients (10.3%) in the navigation group who developed a new pituitary deficit in 1 or more pituitary axes without significant difference between the two groups (Table 3). There were no patients with panhypopituitarism. Generally, the changes to the endocrine functions that were altered postoperatively were transient.
There was no significant difference between the two groups in postoperative DI, both transit (P=0.732) and permanent (P=1.00). There were 6 patients (19.3%) who had DI postoperatively; a permanent condition did occur in 2 patients in the standard group and required medication. In contrast, DI occurred in 4 patients (13.8%) in the navigation group, but was permanent in only 1 patient.
Factors associated with tumor GTR
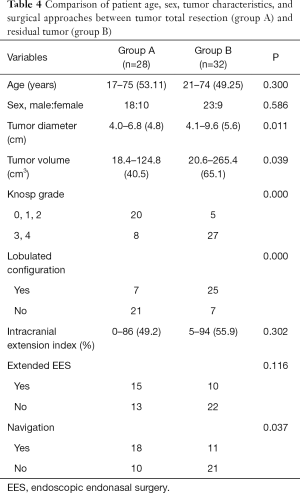
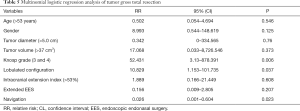
To explore the risk factors associated with tumor GTR, 60 patients were classified into two subgroups according to the extent of resection: subgroup A including 28 patients (46.67%) achieved GTR, and subgroup B, including 32 patients (53.33%) had residual tumor. The extent of resection in the two groups did not significantly differ in terms of patient age, sex, intracranial extension index, and the use of extended EES (Table 4). For patients in subgroup B, occurrence of residual tumors was significantly associated with a larger maximum tumor diameter (mean 5.6 cm; P=0.011) and tumor volume (mean 65.1 cm3; P=0.039), higher Knosp grade (27/32 with grade 3 or grade 4; P=0.000), lobulated tumor configuration (25/32; P=0.000) and lack of intraoperative navigation (21/32; P=0.037) (Table 4). In a multinomial logistic regression, the relative risk for Knosp grade 3 and grade 4 to Knosp grade 0–2 was 52.431 [95% confidence interval (CI): 3.13 to 878.391, P=0.006], from lobulated tumor configuration to no lobulated tumor configuration it was 10.829 (95% CI: 1.153 to 101.735, P=0.037), and from multimode navigation to without navigation it was 0.026 (95% CI: 0.001 to 0.604, P=0.023) (Table 5).
Full table
Full table
Discussion
In the present series, 60 consecutive patients who received EES for GPA by the same senior surgeon were retrospectively reviewed. Based on whether intraoperative multimodal navigation was applied, patients were divided into two groups. Preoperative patient characteristics, clinical outcomes, the extent of resection, residual tumor volume, and complications were statistically compared between the two groups. By using intraoperative multimodal navigation, a much higher GTR, and lower residual tumor volume was achieved, while the clinical outcomes and complications were similar. A multinomial logistic regression model was used to explore the risk factors associated with tumor GTR. Higher Knosp grade of tumor, lobulated tumor configuration, and lack of intraoperative multimodal navigation were relative risk factors associated with GTR. Therefore, intraoperative multimodal navigation appeared to benefit surgical management of GPA by EES. This study is one of the few reports that show the efficacy and the safety of using intraoperative multimodal navigation in EES for GPA. A relatively high GTR for these challenging tumors was achieved in the present series.
GPAs are mostly histological benign, slow-growing, and nonfunctional. Surgical resection remains the main treatment option for GPA. Due to the wide panoramic, up-close visualization of the endoscope, EES has been the primary approach for sellar and parasellar lesions (22,23). Some adenomas that had been considered previously not manageable by EES, such as suprasellar-extended large adenomas with an hourglass constriction, can be endoscopically treated with success and by extended approaches (3,23). The superiority of EES over open transcranial surgery in achieving the GTR of adenomas, with lower perioperative mortality and rate of recurrence, has been reported (4). Nevertheless, surgery in patients with GPA is challenging, and the GTR is still low. On the one hand, it is not uncommon that incomplete resection of GPA may cause postoperative critical apoplexy in which bleeding occurs within the confines of the residual tumors. On the other hand, the recurrence rate of the GPA after its radical resection is low (5). Consequently, the surgical goal of GPA should be a safe and maximum tumor resection depending on the tumor characteristics and the patient.
In general, GPA with a smooth configuration and without massive intracranial extension and cavernous sinus invasion can be resected effectively and safely by EES. However, there are several factors that limit the radical resection of GPA. In evaluating endoscopic surgery for “big” adenomas, Cappabianca et al. (24) noted that “size does not matter”; instead, attention should mostly be paid to the pattern of intracranial growth. Goel proposed a classification system of giant pituitary tumors that assists in indicating the nature of anatomic extensions of the tumor, ease of surgical resection, and possibilities of complete resection, in addition to assessing the need for adjuvant treatment and predicting long-term outcomes (5). According to the classification system, grade I tumors, those that do not invade into the cavernous sinus, can be resected radically and completely, while the resection of tumors within the cavernous sinus in grade II and grade III tumors is less straightforward. Grade IV tumors are those that transgress the diaphragma sella boundary and extend into the subarachnoid spaces of the brain, which are relatively rare, but a challenging clinical problem; only some of these tumors can be resected radically. Similarly, according to Koutourousiou et al. (3), the true limitations of EES are tumors with a mulitlobular configuration and extensions beyond the lateral wall of the CS. Consequently, the radical resection rate of GPA is still low. Our findings were in reasonable agreement with these previous studies in that CS invasion, and lobulated tumor configuration were the predictors of limited the radical resection. Furthermore, we found that the lack of intraoperative multimodal navigation during EES was another independent risk factor for radical resection.
In the present series, GTR was achieved in 28 patients (46.67%), overall, and the GTR reached 62.07% when the multimode navigation system was introduced. In the literature, Elshazly et al. (25) retrospectively reported a 55-case series with GPA, in which GTR was achieved in 24 patients (44%). In their study, neuromonitoring, consisting of motor and somatosensory evoked potentials, was generally used in giant adenomas with vascular involvement. Moreover, Nakao and Itakura (14) presented a total of 43 consecutive patients with pituitary adenomas with a suprasellar extension of >20 mm who underwent tumor resection with a purely endoscopic endonasal approach; GTR was achieved in 20 out of 43 patients (46.51%). They used a navigation system to confirm anatomical landmarks and tumor location. Our overall GTR in the present series was comparable with previously reported series where the GTR ranged from 21.1% to 46.5% (13,14,25,26); however, in our study, the GTR significantly improved when intraoperative navigation was used.
There is little doubt that the marked cavernous sinus invasion is a definitive factor that limits total tumor resection (27). A surgical navigation system, mini-Doppler, and an eye movement monitoring device may help to achieve safe and maximum removal of these tumors (28). It was important to use intraoperative Doppler probe and image guidance to identify the ICA trajectory and reduce the risk of ICA injury (17). Navigation was highly informative in terms of the lateral extent of bone removal at the sellar floor and the limits of lateral explorations, especially in cases of CS involvement, and could be extremely helpful for the protection of the ICA during tumor resection (20). Consistent with previous study, in our present study, the implementation of multimode navigation improved the GTR, reduced the residual tumor volume, and did not increase complication incidence. Although the application of intraoperative multimodal navigation was not random, it seemed to be more frequently used in Knosp grade 4 and recurrent GPA in which GTR was more difficult to achieve; however, the reliability of the conclusion was unaffected.
The effectiveness and utility of the navigation system have been proven, particularly in determining the boundaries between lesions and normal and eloquent structures (17,28). It has a similarly important utility in EES and also aids in the prevention of iatrogenic neurovascular injuries during operation. More modern navigation techniques have been introduced; for example, Micko et al. (29) reported an advanced image guidance protocol that extracted information from multiple modalities and formed them into a single image that included fine sinunasal structures and arteries which could intraoperatively visualize the fine sinunasal sinus structures and small arteries with a high degree of detail. This may help to reduce the rate of complications in endoscopic trans-sphenoidal surgery. However, to the best of our knowledge, there has been a lack of credible evidence suggesting that implementing navigation in EES for GPA can improve surgical outcomes. It is undeniable that navigation cannot precisely displace borders of lesions and normal structures in some cases because the superior and lateral boundaries of GPA are usually surrounded by soft tissues that can drop and displace during tumor evacuation. Although it is not widely available, intraoperative MRI seems to be the best modality for evaluation of residual tumors (30). There are at least two reasons why the application of intraoperative multimodal navigation may facilitate improving the GTR and reduce residual tumor volume in EES for GPA. One is that the operation seems more “aggressive” when applied and takes advantage of the intraoperative navigation system and Doppler probe. Although there was no significant difference in the use of extended EES between the two groups in our study, it seemed to be more frequently used in extended EES in the navigation group. Furthermore, the bone overlying the sellar and parasellar region was more frequently removed in the navigation group than in the standard group, especially in the cases of CS involvement and extension into the anterior cranial fossa. The other reason is that the navigation system is used to evaluate the extent of resection whenever necessary which can minimize “unexpected” residual tumors and improve the rate of GTR.
The relatively small number of cases for each group and its retrospective design were the major limitations of the current study. Furthermore, a selection bias could have been present because the use of intraoperative multimode navigation was not random. Multimode navigation seemed to be more frequently used in Knosp grade 4 and recurrent tumors. It is worth noting the possible biases that there were more recurrent tumors in the navigation group than those in the standard group. Multimode navigation might be extremely useful in recurrent cases, as GTR of these tumors seems to be more challenging. Indeed, the use of navigation yielded more GTRs and lowered residual tumor volume which further confirms the value of applying navigation during EES for GPA. Further studies of a larger sample size and longer follow-up are necessary to validate the effectiveness and superiority of intraoperative multimode navigation in EES for GPA.
Conclusions
In the current series of GPA, the use of intraoperative multimode navigation in EES yielded more GTRs and lower residual tumor volume. Therefore, intraoperative multimode navigation appears to be safe and effective in EES for GPA. In particular, it is recommended that intraoperative multimode navigation be used for the more “aggressive” and recurrent GPA.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81701304), the China Postdoctoral Science Foundation (grant No. 2017M623432), and the Shaanxi Province General Project (No. 2017SF-150 and 2017SF-176).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work (if applied, including full data access, the integrity of the data, and the accuracy of the data analysis) in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional ethics committee of Tangdu Hospital (No. TDLL-2011034). Given the study design of a retrospective chart review, patient consent was not required.
References
- Gondim JA, Almeida JP, Albuquerque LA, et al. Giant pituitary adenomas: surgical outcomes of 50 cases operated on by the endonasal endoscopic approach. World Neurosurg 2014;82:e281-90. [Crossref] [PubMed]
- Agrawal A, Cincu R, Goel A. Current concepts and controversies in the management of non-functioning giant pituitary macroadenomas. Clin Neurol Neurosurg 2007;109:645-50. [Crossref] [PubMed]
- Koutourousiou M, Gardner PA, Fernandez-Miranda JC, et al. Endoscopic endonasal surgery for giant pituitary adenomas: advantages and limitations. J Neurosurg 2013;118:621-31. [Crossref] [PubMed]
- Komotar RJ, Starke RM, Raper DM, et al. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary 2012;15:150-9. [Crossref] [PubMed]
- Goel A. Challenge of giant pituitary tumors. World Neurosurg 2014;82:e121-4. [Crossref] [PubMed]
- Kuga D, Toda M, Ozawa H, et al. Endoscopic Endonasal Approach Combined with a Simultaneous Transcranial Approach for Giant Pituitary Tumors. World Neurosurg 2019;121:173-9. [Crossref] [PubMed]
- Yano S, Hide T, Shinojima N, et al. Endoscopic endonasal skull base approach for parasellar lesions: Initial experiences, results, efficacy, and complications. Surg Neurol Int 2014;5:51. [Crossref] [PubMed]
- Chabot JD, Chakraborty S, Imbarrato G, et al. Evaluation of Outcomes After Endoscopic Endonasal Surgery for Large and Giant Pituitary Macroadenoma: A Retrospective Review of 39 Consecutive Patients. World Neurosurg 2015;84:978-88. [Crossref] [PubMed]
- Dehdashti AR, Ganna A, Karabatsou K, et al. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery 2008;62:1006-15; discussion 1015-7. [Crossref] [PubMed]
- Jho HD, Carrau RL, Ko Y, et al. Endoscopic pituitary surgery: an early experience. Surg Neurol 1997;47:213-22; discussion 222-3. [Crossref] [PubMed]
- Juraschka K, Khan OH, Godoy BL, et al. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J Neurosurg 2014;121:75-83. [Crossref] [PubMed]
- Paluzzi A, Fernandez-Miranda JC, Tonya SS, et al. Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary 2014;17:307-19. [Crossref] [PubMed]
- Kuo CH, Yen YS, Wu JC, et al. Primary Endoscopic Transnasal Transsphenoidal Surgery for Giant Pituitary Adenoma. World Neurosurg 2016;91:121-8. [Crossref] [PubMed]
- Nakao N, Itakura T. Surgical outcome of the endoscopic endonasal approach for non-functioning giant pituitary adenoma. J Clin Neurosci 2011;18:71-5. [Crossref] [PubMed]
- Frank G, Pasquini E, Farneti G, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology 2006;83:240-8. [Crossref] [PubMed]
- Knosp E, Kitz K, Steiner E, et al. Pituitary adenomas with parasellar invasion. Acta Neurochir Suppl (Wien) 1991;53:65-71. [Crossref] [PubMed]
- Cohen-Cohen S, Gardner PA, Alves-Belo JT, et al. The medial wall of the cavernous sinus. Part 2: Selective medial wall resection in 50 pituitary adenoma patients. J Neurosurg 2018;1:1-10. [PubMed]
- Goel A, Nadkarni T, Muzumdar D, et al. Giant pituitary tumors: a study based on surgical treatment of 118 cases. Surg Neurol 2004;61:436-45; discussion 445-6. [Crossref] [PubMed]
- Goel A, Deogaonkar M, Desai K. Fatal postoperative 'pituitary apoplexy': its cause and management. Br J Neurosurg 1995;9:37-40. [Crossref] [PubMed]
- Dolati P, Eichberg D, Golby A, et al. Multimodal Navigation in Endoscopic Transsphenoidal Resection of Pituitary Tumors Using Image-Based Vascular and Cranial Nerve Segmentation: A Prospective Validation Study. World Neurosurg 2016;95:406-13. [Crossref] [PubMed]
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006;116:1882-6. [Crossref] [PubMed]
- Nishioka H. Recent Evolution of Endoscopic Endonasal Surgery for Treatment of Pituitary Adenomas. Neurol Med Chir (Tokyo) 2017;57:151-8. [Crossref] [PubMed]
- Singh H, Essayed WI, Cohen-Gadol A, et al. Resection of pituitary tumors: endoscopic versus microscopic. J Neurooncol 2016;130:309-17. [Crossref] [PubMed]
- Cappabianca P, Cavallo LM, Solari D, et al. Size does not matter. The intrigue of giant adenomas: a true surgical challenge. Acta Neurochir (Wien) 2014;156:2217-20. [Crossref] [PubMed]
- Elshazly K, Kshettry VR, Farrell CJ, et al. Clinical Outcomes After Endoscopic Endonasal Resection of Giant Pituitary Adenomas. World Neurosurg 2018;114:e447-56. [Crossref] [PubMed]
- Azab WA, Nasim K, Abdelnabi EA, et al. Endoscopic Endonasal Excision of Large and Giant Pituitary Adenomas: Radiological and Intraoperative Correlates of the Extent of Resection. World Neurosurg 2019;126:e793-802. [Crossref] [PubMed]
- Nishioka H, Hara T, Nagata Y, et al. Inherent Tumor Characteristics That Limit Effective and Safe Resection of Giant Nonfunctioning Pituitary Adenomas. World Neurosurg 2017;106:645-52. [Crossref] [PubMed]
- Nishioka H, Fukuhara N, Horiguchi K, et al. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J Neurosurg 2014;121:505-10. [Crossref] [PubMed]
- Micko A, Hosmann A, Wurzer A, et al. An advanced protocol for intraoperative visualization of sinunasal structures: experiences from pituitary surgery. J Neurosurg 2019;31:1-9. [PubMed]
- Pal’a A, Knoll A, Brand C, et al. The Value of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microsurgical Transsphenoidal Pituitary Adenoma Resection. World Neurosurg 2017;102:144-50. [Crossref] [PubMed]


