
Cardiovascular risk and mortality in patients with active and treated hypercortisolism
Introduction
Cushing syndrome (CS) is a rare endocrine disorder caused by chronic exposure to endogenous cortisol hypersecretion or exogenous administration of glucocorticoids (1). The estimated incidence of endogenous CS is approximately 0.2 to 5.0 cases per million per year with an estimated prevalence of 39 to 79 cases per million in various populations (2-5). CS presents with a wide spectrum of clinical manifestations, which include physical signs such as central obesity, rounded face, facial plethora, dorsocervical and supraclavicular fat pads, striae, thinning of the skin and easy bruising (1), as well as metabolic manifestations, such as hypertension, diabetes mellitus, and dyslipidemia (6,7). The median age of diagnosis with CS is 41.4 years, and women are more frequently affected than men (female-to-male ratio of 3:1) (2-5). Among the different etiologies of CS, adrenocorticotropic hormone (ACTH)-dependent CS is most common affecting around 80% of patients, usually caused by a pituitary adenoma producing ACTH (also called Cushing disease, CD). Rarely, it may be caused by ectopic ACTH or corticotropin-releasing hormone production (ectopic CS) (4,7,8). ACTH-independent CS occurs in approximately 20% of patients, and is caused by autonomous cortisol production from an adrenal cortical adenoma, macronodular or micronodular hyperplasia, or adrenal cortical carcinoma (2,9,10).
Mild autonomous cortisol secretion (MACS) affects around 30% of patients with adrenal tumors (11,12) and is characterized by the presence of metabolic abnormalities in the absence of overt clinical features of CS (12-14). Patients with MACS have been reported to have increased risk of cardiovascular events and higher mortality than those with nonfunctional tumors (6,15). As surgery is not uniformly performed in patients with MACS, these patients are more likely to have longstanding, though mild hypercortisolism.
Untreated hypercortisolism is associated with increased mortality and morbidity, with increase prevalence of cardiovascular events, sepsis, and thromboembolism as the leading causes of death (16). Surgery is the treatment of choice in all subtypes of endogenous CS. If surgery is not possible, medical therapy and radiotherapy may be occasionally used (17). Improvement or resolution of hypercortisolism-induced comorbidities occurs with surgery; however the degree of improvement varies. In this review, we will describe the prevalence and mechanisms behind cardiovascular comorbidities, as well as mortality in patients with untreated hypercortisolism. In addition, we will summarize the effect of therapy on cardiovascular risk factors, cardiovascular events, and mortality.
Hypertension in hypercortisolism
At the time of diagnosis, around 37–82% of patients with CS and 58–64% of patients with MACS present with hypertension (Table 1). Both duration and severity of untreated hypercortisolism contribute to the development of hypertension (5,24). Prevalence, as well as degree of hypertension is higher in patients with ACTH-independent CS when compared to patients with CD, likely due to the differences in demographics and duration of hypercortisolism (5,25-27). The majority of patients with hypertension at baseline are treated with none or one antihypertensive agent, and around a third of patients require 2 or more medications (2,28).
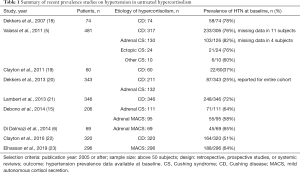
Full table
Hypercortisolism-induced hypertension is a multi-factorial disease that mainly involves activation of the mineralocorticoid and glucocorticoid receptors, renin-angiotensin system, sympathetic nervous system and an impaired balance between vasodilators and vasoconstrictors (Table 2). Aldosterone and cortisol have a similar affinity at the level of mineralocorticoid receptors which are mainly expressed in the kidneys (29). The corticosteroid pathway enzyme 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) is the main regulator of the binding selectivity. It acts by catalyzing the deactivation of cortisol into cortisone (40). In the setting of hypercortisolism, the 11beta-HSD2 capacity to deactivate cortisol is overwhelmed, making cortisol available to act on the mineralocorticoid receptor. This leads to retention of sodium and water in exchange for potassium, causing hypertension and hypokalemia, especially in patients with extremely high cortisol concentrations (41,42). Binding of cortisol to the glucocorticoid receptor also contributes to the development of hypertension by enhancing epithelial sodium channel activation and glomerular hyperfiltration (30), and thus spironolactone, a mineralocorticoid receptor antagonist may not be effective as a single agent to normalize blood pressure in hypercortisolism-induced hypertension.

Full table
Another mechanism for hypercortisolism-induced hypertension involves the renin-angiotensin system. Hypercortisolism leads to increased hepatic production of angiotensinogen (26,31). Renin is responsible for the conversion of angiotensinogen to angiotensin I, which is then converted to angiotensin II by angiotensin-converting-enzyme. Angiotensin II is a vasoconstrictor, and it also stimulates the production of aldosterone that in turn leads to water retention and sodium reabsorption. In hypercortisolism, renin is suppressed or normal (26,31). Despite the increase of angiotensinogen, the concentrations of circulating angiotensin II in patients with hypercortisolism is reportedly unchanged (32). However, there is upregulation of angiotensin II (Type 1A) receptors, causing an enhanced response to angiotensin II with vasoconstriction (32). Several studies showed that oral administration of the angiotensin-converting-enzyme-inhibitor in patients with hypercortisolism leads to blood pressure improvement (26,43).
Lastly, several vasoactive substances have been reported to contribute to the pathogenesis of cortisol induced hypertension, including endothelin-1, erythropoietin, nitric oxide, prostaglandins, prostacyclins, and the kallikrein-kinin system. Endothelin-1 (a vasoconstrictor) concentrations are increased in patients with hypercortisolism, possibly related to the cortisol-induced endothelial damage and increased vascular permeability (34). Hypercortisolism inhibits the gene expression of nitric oxide synthase and thus leads to decreased concentrations of nitric oxide, a potent vasodilator (36,37,44). Prostaglandin E2 and I2 are two important prostaglandins with strong vasodilatory effects that are inhibited by hypercortisolism through induction of lipomodulin synthesis (26,38,45). In addition, hypercortisolism was associated with low concentrations of urinary kallikrein, another vasodilator, likely due to the inhibitory effect of cortisol (26,46).
Abnormal glucose metabolism in hypercortisolism
Abnormal glucose metabolism has been reported in 14–74% of patients with untreated hypercortisolism, with impaired fasting glucose reported in 21–64% and diabetes mellitus in 21–47% of patients (Table 3). Prevalence of glucose abnormalities varies based on the etiology of hypercortisolism. Hyperglycemia in patients with ectopic CS is inconsistently reported with prevalence of as low as 38% and as high as 74% (27,48). Patients with CD have a prevalence of diabetes mellitus between 18% and 36% and in patients with adrenal CS, prevalence of diabetes mellitus at the time of diagnosis ranges between 34% and 41% (Table 3). In patients with MACS, prevalence of diabetes mellitus varies between 18% and 30% at the time of initial diagnosis, with another 5% of patients developing new-onset diabetes mellitus during follow-up (6,23,49). As reported in two studies, the majority of patients with hyperglycemia in the setting of CS are not treated with any medications or take oral hypoglycemic agents only; however, around a quarter of patients are treated with insulin at the time of diagnosis (2,28).
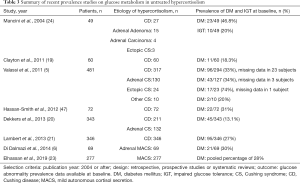
Full table
The mechanisms of glucose dysregulation in hypercortisolism include stimulation of liver gluconeogenesis (50), interference with glucose transporters leading to increased hepatic and peripheral insulin resistance (51), increased hepatic lipogenesis contributing to steatosis (52), and lastly detrimental effect on pancreatic insulin secretion (53,54). Hypercortisolism-induced visceral adiposity also contributes to glucose dysregulation. The visceral fat accumulation caused by hypercortisolism is hypothesized to occur due to higher expression of 11β-HSD1 in the visceral adipose tissue, allowing higher rates of cortisone conversion to active cortisol (55).
Dyslipidemia in hypercortisolism
The prevalence of dyslipidemia in untreated overt hypercortisolism varies between 12–72% (2,21,27,56). In a population-based study of 190 patients with hypercortisolism and available data at baseline, dyslipidemia was noted in 28% of patients (2). In a smaller study but with more in-depth assessment, dyslipidemia was demonstrated in 64% of patients with CD and 53% of patients with adrenal CS (27). Another larger retrospective study of 346 patients with CS reported dyslipidemia in only 16% of patients (21). Patients with MACS present with a 26–34% prevalence of dyslipidemia, similar to overt hypercortisolism, likely due to a longer duration of untreated but mild hypercortisolism (23,57). With follow-up, an additional 6% of patients were reported to develop dyslipidemia (23). Dyslipidemia in hypercortisolism usually presents with low HDL and elevated LDL and triglyceride concentrations (2,27).
Several mechanisms of hypercortisolism-induced dyslipidemia have been described. Hypercortisolism promotes lipolysis (58) with subsequent increase of free fatty acids leading to accumulation of hepatic fat, reduction of glucose uptake and insulin signaling (59). Other mechanisms include hepatic overexpression of 11β-HSD1 and polymorphisms at the level of glucocorticoid receptor in the liver, both contributing to the development of hepatic steatosis (60,61). Hepatic steatosis is evident in at least 20% of patients, as was demonstrated on ultrasound in a study of 50 patients with overt CS of various etiologies (62).
Cardiovascular events in hypercortisolism
Patients with hypercortisolism demonstrate high rates of cardiovascular risk factors, cardiac remodeling, dysfunction and vascular atherosclerosis, which contribute to high rates of cardiovascular events (24,63). Left ventricular hypertrophy, decrease in systolic strain and impaired relaxation pattern with a decrease in diastolic filling have been reported in several studies (63-65). In patients with CS, left ventricular enlargement was reported to be more pronounced when compared to patients with hypertension without hypercortisolism, suggesting that factors other than elevated blood pressure contribute to cardiac dysfunction (64). Possible mechanisms include an enhanced response to angiotensin II (32) and hypercortisolism induced activation of the mineralocorticoid receptor (42) leading to myocardial fibrosis, ventricular remodeling, impairment of relaxation and consequently, the development of heart failure (66,67). Additionally, intimal media thickness of common carotid artery, aorta and femoral artery is significantly increased in patients with CS when compared to healthy controls matched for other cardiovascular risk factors (56,68). Vascular damage with increased formation of atherosclerotic plaques has been reported in patients with CS (56,68) and could explain the increased risk of myocardial infarction and stroke reported in patients with CS (20).
Patients with both CS and MACS demonstrate an increased risk of cardiovascular events, including coronary artery disease, myocardial infarction, stroke, transient ischemic attack, and heart failure (6,20,28,57). In a study of 343 patients with overt hypercortisolism, the risk for myocardial infarction, stroke, and cardiac failure was high with an age and sex adjusted hazard ratio of 2.2 to 6.8 (20). In patients with persistent CS, rate of cardiovascular events was reported to be as high as 29% (28). In 69 patients with MACS, baseline prevalence of coronary heart disease and stroke was 10% and 7%, respectively (6). During a median follow-up of 7.5 years, patients with MACS demonstrated a higher incidence of cardiovascular events when compared to patients without MACS (16.7% vs. 6.7%), with an unadjusted hazard ratio for new cardiovascular events of 3 (6). History of cardiovascular events was reported in 6.3% of patients with MACS at the time of diagnosis, and 15.5% more patients developed new cardiovascular events during 5-year follow-up, which was three times higher than patients without MACS (57).
Treatment of hypercortisolism
Surgery is the treatment of choice in overt hypercortisolism of any etiology, while other therapies, such as radiation therapy (for pituitary tumors) or medical therapy are reserved only when surgery is not possible or not curative (17). Surgical options include transsphenoidal selective tumor resection in CD, laparoscopic unilateral adrenalectomy in adrenal CS, and resection of ACTH-secreting neuroendocrine tumor in ectopic CS (17). Bilateral adrenalectomy may be performed in patients with macronodular and micronodular adrenal hyperplasia presenting with ACTH-independent CS or patients with ACTH-dependent Cushing syndrome where surgical cure is not possible (17). In patients with MACS, adrenalectomy is usually considered for patients with hypercortisolism-induced comorbidities such as hypertension, obesity, diabetes mellitus, dyslipidemia and low bone mass (11,69). Medical therapy includes agents that modulate ACTH release (somatostatin analogs, dopamine agonists), agents that inhibit steroidogenesis (ketoconazole, metyrapone, mitotane, etomidate), and agents that block the glucocorticoid receptor (mifepristone) (17). Medical therapy can be used as monotherapy or in combination, usually as a temporary action to quickly achieve eucortisolism to prevent acute complications, when the surgery has to be delayed (17,70).
Improvement of comorbidities following remission of hypercortisolism
Effect of therapy for hypercortisolism on hypertension
Resolution of hypercortisolism improves blood pressure control in up to 30–70% of patients (Tables 4,5). Patients may continue to have persistent hypertension due to cortisol induced irreversible structural cardiovascular changes and vascular remodeling (84), as well as left ventricular hypertrophy (55,85).
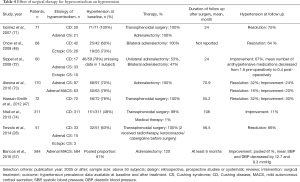
Full table
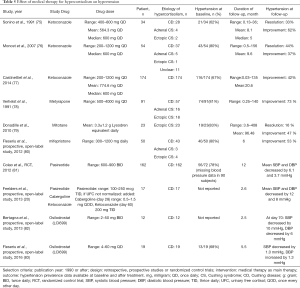
Full table
It is important to note that studies reporting on improvement in hypertension after surgery or with medical therapy are inconsistent in their definitions of improvement (Tables 4,5). In patients with CD after a curative transsphenoidal surgery, multiple studies reported persistent hypertension in 11–40% of the cases after a follow-up period ranging from 1 to 18 years (56,71,74,86,87). In patients treated with bilateral adrenalectomy for ACTH-dependent hypercortisolism from an occult source, 64% of patients improved blood pressure (48). In a cohort of 60 patients with adrenal CS, hypertension was present in 78% of patients and improved in 67% with adrenalectomy (72). Hypertension is also the most likely comorbidity to improve after adrenalectomy in patients with MACS, reported to improve in 60.5% of patients at a median follow-up of 28 months. The mean decrease in blood pressure measurements after surgery in this study was 12.7 and 9.3 mmHg for systolic and diastolic blood pressure, respectively (57).
Several studies reported on the effectiveness of medical therapy for hypercortisolism on hypertension. Most of these studies were of short duration with a small sample size (Table 5). Ketoconazole, an inhibitor of 17-alpha hydroxilase and 17, 20 lyase has been reported to improve blood pressure in 40–60% of the patients in three studies (75-77). Therapy with metyrapone, an inhibitor of 11-beta-hydroxylase, led to improvement of hypertension in 73% of patients (78). Notably, 5.5% of the patients treated with metyrapone developed hypokalemia due to activation of the mineralocorticoid receptors and all responded well with potassium supplements, amiloride or triamterene (78). Therapy with mifepristone, a glucocorticoid receptor antagonist, was reported to lead to hypertension improvement in up to 52% of patients, but also led to exacerbation of hypertension in 13.5% of patients, likely due to activation of mineralocorticoid receptor (80). Mitotane, an adrenolytic agent used in patients with adrenocortical carcinoma, was reported to improve hypertension in 63% of patients with severe hypercortisolism (79). Etomidate, another steroidogenesis inhibitor, is the only drug with parenteral administration used in patients with hypercortisolism (88). Due to its fast onset of action with half-life of 75 minutes and mode of administration, it is usually reserved for hospitalized patients with acute and/or life-threatening severe hypertension (88). Dopamine agonist therapy with cabergoline and somatostatin analog therapy with pasireotide have been reported to have a minor effect on hypertension in patients with CS with blood pressure decrease of 4–6 mmHg (81,89).
Effect of therapy for hypercortisolism on glucose metabolism
Reversal of hypercortisolism leads to improvement in glucose metabolism (27,28,48,56,72,74,86). Prevalence of diabetes mellitus in patients treated for CS (mostly CD) decreases from 20–47% before treatment to 10–33% (Table 6). In a cohort of patients with predominantly adrenal CS but also CD and ectopic CS, treated with adrenalectomy, resolution of hyperglycemia was observed in 79% of patients (72). While both diabetes and impaired fasting glucose persist after cure from CS, improvement in diabetes control (as defined by hemoglobin A1c, fasting glucose, or reduction in number and dose of medications) is poorly reported in patients with overt hypercortisolism. In patients with MACS, adrenalectomy was reported to lead to diabetes mellitus improvement or resolution in 51.5% of patients (57).
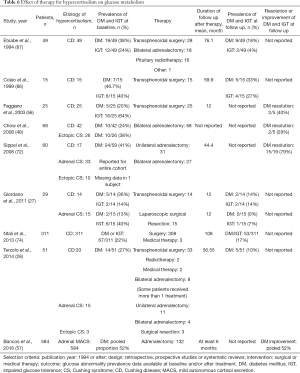
Full table
Pharmacotherapy for hypercortisolism was reported to lead to improvement in glycemic control. Mifepristone is the agent approved for patients with CS and hyperglycemia, which can reduce fasting glucose concentrations by around 40 mg/dL (80). Improvements in glucose control have also been reported with ketoconazole, metyrapone and cabergoline, and the degree of improvement likely depends on the effectiveness of hypercortisolism control. In a study of 38 patients with CD treated with ketoconazole, HbA1c decreased by 0.5–1% after 6–12 months of therapy (90). Three-month therapy with metyrapone in 7 patients with CD and impaired glucose tolerance was reported to lead to normalization of glucose metabolism in 5 patients (91). In another study of 20 patients with CS, 3-month therapy with cabergoline reduced fasting glucose concentrations by 10 mg/dL (92).
Effect of therapy for hypercortisolism on dyslipidemia
Effect of therapy for hypercortisolism on dyslipidemia is variable but usually mild. In a population-based study of 190 patients with CS and available data, prevalence of dyslipidemia at follow-up was similar to baseline (2). No improvement in lipid profile was observed in patients with CD in remission during 5 years of follow-up with total cholesterol and LDL concentrations higher than controls matched for sex and age (86). In another study of 25 patients with CD, LDL concentrations improved only by a mean of 0.6 mmol/L after 1 year of remission (56). Prevalence of dyslipidemia decreased in 15 patients with adrenal CS (from 53% to 27%) and 14 patients with CS (from 64% to 50%) with treatment for hypercortisolism (27). A more significant improvement was reported in a study of 51 patients with CS, when hypercortisolism remission led to normalization of LDL and HDL in 60% and 78% of patients, respectively (28). In patients with MACS, adrenalectomy did not result in statistically significant improvement of dyslipidemia after a median of 28 months following surgery (57).
Effect of therapy for hypercortisolism on cardiovascular events
Remission from CS leads to a decrease in frequency of cardiovascular risk factors and decrease in rate of cardiovascular events (27,28,57). In a study of 51 patients with CS, rate of cardiovascular events in patients with CS in remission was 3.9%, as opposed to 29% in patients with persistent CS (28). However, risk for cardiovascular events remains elevated despite remission when compared to controls, suggesting that achieving eucortisolism does not completely eliminate cardiovascular risk (18,20,93). In a study of 25 patients with CS followed for 1 year after remission, despite improvement in vascular parameters, patients continued to demonstrate increased stiffness of carotid arteries wall and persistence of atherosclerotic plaques (56). In 71 patients with history of CS, structural changes with abnormal left ventricular mass and concentric remodeling were still seen after a median of 46 months following curative surgeries (85). At a median of 11 years after curative therapy, patients with history of CS were reported to have higher prevalence of coronary calcifications and evidence of atheroma plaques on cardiac computed tomography angiograms when compared to controls (94). Of 15 patients with history of CD followed 5 years after curative surgeries, 26.7% had evidence of atherosclerosis based on echo-Doppler ultrasonography (versus 0% in controls matched for age and sex) (86). In a study of 160 patients with CS in remission with at least 3 years of follow-up compared to 879 patients with nonfunctioning pituitary adenoma, the prevalence of cardiovascular disease and cardiovascular risk factors was significantly higher in patients with CS despite remission (93). Risk of myocardial infarction and stroke remained elevated in a population study of 343 patients with CS in remission who were followed for 1–30 years with age and sex adjusted HR of 3.6 and 1.5 respectively (20).
Mortality in patients with hypercortisolism
Mortality in untreated or persistent hypercortisolism
Untreated or persistent hypercortisolism of any cause is associated with increased mortality (Table 7). In a meta-analysis of studies on mortality in CD published in 2015, a pooled standardized mortality ratio (SMR) was 4.6 and the range was between 2.4 and 16 (95). In a more recent study of 40 patients with persistent CS, SMR was 6.9 (96). Causes of death in patients with persistent CD and adrenal CS were reported to be primarily cardiovascular but also from infection or malignancy (74,96).
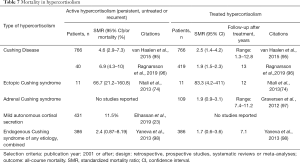
Full table
Mortality is highest in patients with ectopic CS, with SMR of 66.7 if remission is not achieved (74). Causes of death in patients with ectopic CS were reported to be mainly metastatic disease and sepsis, in 40% and 30% of patients respectively (74). In another study of patients with ectopic CS, poor survival was mainly due to cardiovascular (43%) and infectious (29%) causes (98). Patients with small cell lung carcinoma have the worst prognosis, whereas those bronchial carcinoid tumors have better survival (74,99).
Patients with MACS were reported to have a higher mortality, proportional to the severity of hypercortisolism. In a study of 206 patients diagnosed with an adrenal mass followed for a mean of 4.2 years, mortality was reported to be highest in patients with cortisol concentrations following the overnight dexamethasone suppression (DST) >5 mcg/dL (26%), followed by mortality in patients with DST between 1.8 and 5 mcg/dL (13%), as compared to only 1% mortality in patients with DST<1.8 mcg/dL (15). In patients with DST >1.8 mcg/dL, primary cause of death was cardiovascular (50%), followed by respiratory and infectious causes of death (33%) (15). A recent meta-analysis of patients with adrenal adenomas reported that mortality in patients with MACS is similar to patients with nonfunctioning adenomas at 11%, despite higher rate of new cardiovascular events in patients with MACS. It is important to note that very few studies reported on mortality of patients with untreated MACS, and duration of follow up was insufficient for confident conclusions (23).
Mortality in cured hypercortisolism
Mortality in patients with hypercortisolism in remission is lower when compared to patients with persistent hypercortisolism, but is still increased with a pooled SMR of 2.5, as reported in a meta-analysis of studies of patients with CD (95) (Table 7). A similar finding was reported in a multicenter study in the UK, which found that those patients with CD in remission for more than 10 years still had increased risk of mortality (22). In a more recent study of 419 patients with CD and confirmed remission, SMR was 1.9 (96) with increased mortality mainly due to cardiovascular causes. Scarce data exist on mortality in treated adrenal CS. A meta-analysis of several studies on mortality in patients with treated adrenal CS demonstrated no statistically significant increase in mortality (97).
Possible contributors to persistent mortality after cure include co-existing pituitary deficiency (especially secondary adrenal insufficiency), prolonged duration of hypercortisolism leading to irreversibility of some of the metabolic consequences, persistence of pro-inflammatory cytokines and left ventricular hypertrophy (19,21,63,96,100). Older age at the time of diagnosis and time in remission were significantly associated with mortality (96). Another study reported that older age, male sex, and depression predicted mortality in patients with CD in remission (21). Post-treatment pituitary dysfunction in patients with CD was also demonstrated to play a role in mortality despite correction of hypercortisolism (96). For example, glucocorticoid replacement therapy was associated with HR of 2.6 for mortality, while levothyroxine and growth hormone supplementation demonstrated a HR of 1.2 and 0.4, respectively (96). Treatment modality for CD also plays a role in mortality. For example, in 102 patients with CD who were treated with bilateral adrenalectomy, SMR was 2.7; whereas in patients treated with pituitary surgery or radiotherapy SMR did not increase (96). Presence of hypertension and diabetes mellitus was associated with higher mortality in one study (19), but not demonstrated in another study (96).
Conclusions
Patients with untreated overt CS and MACS present with high prevalence of cardiovascular risk factors such as hypertension, abnormal glucose metabolism, dyslipidemia, and ultimately a high rate of cardiovascular events and increased mortality. Treatment of hypercortisolism is primarily surgical, although medical therapy or radiation may occasionally be used with the goal to achieve eucortisolism. Optimal treatment can lead to improvement or reversal of cardiovascular risk factors and decrease the incidence of new cardiovascular events. Even with successful treatment of hypercortisolism, patients in remission continue to demonstrate higher mortality than the general population.
Acknowledgments
Funding: James A. Ruppe Career Development Award in Endocrinology to I. Bancos, Robert and Elizabeth Strickland Career Development Award within the Division of Endocrinology, Metabolism, Diabetes and Nutrition to I. Bancos, Advancement in Medicine Catalyst award to I. Bancos.
Footnote
Conflicts of Interest: IB served as consultant on advisory board of HRA Pharma. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. [Crossref] [PubMed]
- Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing's syndrome in New Zealand. Clin Endocrinol (Oxf) 2011;75:436-42. [Crossref] [PubMed]
- Lindholm J, Juul S, Jorgensen JO, et al. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab 2001;86:117-23. [PubMed]
- Steffensen C, Bak AM, Rubeck KZ, et al. Epidemiology of Cushing's syndrome. Neuroendocrinology 2010;92 Suppl 1:1-5. [Crossref] [PubMed]
- Valassi E, Santos A, Yaneva M, et al. The European Registry on Cushing's syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol 2011;165:383-92. [Crossref] [PubMed]
- Di Dalmazi G, Berr CM, Fassnacht M, et al. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing's syndrome: a systematic review of the literature. J Clin Endocrinol Metab 2014;99:2637-45. [Crossref] [PubMed]
- Pivonello R, De Martino MC, De Leo M, et al. Cushing's Syndrome. Endocrinol Metab Clin North Am 2008;37:135-49. ix. [Crossref] [PubMed]
- Newell-Price J, Bertagna X, Grossman AB, et al. Cushing's syndrome. Lancet 2006;367:1605-17. [Crossref] [PubMed]
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2003;88:5593-602. [Crossref] [PubMed]
- Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin- independent Cushing syndrome). Endocr Dev 2008;13:117-32. [Crossref] [PubMed]
- Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1-G34. [Crossref] [PubMed]
- Terzolo M, Stigliano A, Chiodini I, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol 2011;164:851-70. [Crossref] [PubMed]
- Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab 2011;96:1223-36. [Crossref] [PubMed]
- Di Dalmazi G, Pasquali R, Beuschlein F, et al. Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur J Endocrinol 2015;173:M61-71. [Crossref] [PubMed]
- Debono M, Bradburn M, Bull M, et al. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab 2014;99:4462-70. [Crossref] [PubMed]
- Javanmard P, Duan D, Geer EB. Mortality in Patients with Endogenous Cushing's Syndrome. Endocrinol Metab Clin North Am 2018;47:313-33. [Crossref] [PubMed]
- Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:2807-31. [Crossref] [PubMed]
- Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in patients treated for Cushing's disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab 2007;92:976-81. [Crossref] [PubMed]
- Clayton RN, Raskauskiene D, Reulen RC, et al. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab 2011;96:632-42. [Crossref] [PubMed]
- Dekkers OM, Horvath-Puho E, Jorgensen JO, et al. Multisystem morbidity and mortality in Cushing's syndrome: a cohort study. J Clin Endocrinol Metab 2013;98:2277-84. [Crossref] [PubMed]
- Lambert JK, Goldberg L, Fayngold S, et al. Predictors of mortality and long-term outcomes in treated Cushing's disease: a study of 346 patients. J Clin Endocrinol Metab 2013;98:1022-30. [Crossref] [PubMed]
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing's disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016;4:569-76. [Crossref] [PubMed]
- Elhassan YS, Alahdab F, Prete A, et al. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Ann Intern Med 2019;171:107-16. [Crossref] [PubMed]
- Mancini T, Kola B, Mantero F, et al. High cardiovascular risk in patients with Cushing's syndrome according to 1999 WHO/ISH guidelines. Clin Endocrinol (Oxf) 2004;61:768-77. [Crossref] [PubMed]
- Imai Y, Abe K, Sasaki S, et al. Altered circadian blood pressure rhythm in patients with Cushing's syndrome. Hypertension 1988;12:11-9. [Crossref] [PubMed]
- Saruta T, Suzuki H, Handa M, et al. Multiple factors contribute to the pathogenesis of hypertension in Cushing's syndrome. J Clin Endocrinol Metab 1986;62:275-9. [Crossref] [PubMed]
- Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing's syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf) 2011;75:354-60. [Crossref] [PubMed]
- Terzolo M, Allasino B, Pia A, et al. Surgical remission of Cushing's syndrome reduces cardiovascular risk. Eur J Endocrinol 2014;171:127-36. [Crossref] [PubMed]
- Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension 2005;46:1227-35. [Crossref] [PubMed]
- Bailey MA, Mullins JJ, Kenyon CJ. Mineralocorticoid and glucocorticoid receptors stimulate epithelial sodium channel activity in a mouse model of Cushing syndrome. Hypertension 2009;54:890-6. [Crossref] [PubMed]
- Connell JM, Whitworth JA, Davies DL, et al. Effects of ACTH and cortisol administration on blood pressure, electrolyte metabolism, atrial natriuretic peptide and renal function in normal man. J Hypertens 1987;5:425-33. [Crossref] [PubMed]
- Ritchie CM, Sheridan B, Fraser R, et al. Studies on the pathogenesis of hypertension in Cushing's disease and acromegaly. Q J Med 1990;76:855-67. [PubMed]
- Heaney AP, Hunter SJ, Sheridan B, et al. Increased pressor response to noradrenaline in pituitary dependent Cushing's syndrome. Clin Endocrinol (Oxf) 1999;51:293-9. [Crossref] [PubMed]
- Kirilov G, Tomova A, Dakovska L, et al. Elevated plasma endothelin as an additional cardiovascular risk factor in patients with Cushing's syndrome. Eur J Endocrinol 2003;149:549-53. [Crossref] [PubMed]
- Kelly JJ, Martin A, Whitworth JA. Role of erythropoietin in cortisol-induced hypertension. J Hum Hypertens 2000;14:195-8. [Crossref] [PubMed]
- Wallerath T, Witte K, Schafer SC, et al. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci U S A 1999;96:13357-62. [Crossref] [PubMed]
- Kelly JJ, Tam SH, Williamson PM, et al. The nitric oxide system and cortisol-induced hypertension in humans. Clin Exp Pharmacol Physiol 1998;25:945-6. [Crossref] [PubMed]
- Axelrod L. Inhibition of prostacyclin production mediates permissive effect of glucocorticoids on vascular tone. Perturbations of this mechanism contribute to pathogenesis of Cushing's syndrome and Addison's disease. Lancet 1983;1:904-6. [Crossref] [PubMed]
- Shimamoto K, Ura N, Nomura N, et al. Significance of renal kininases in patients with Cushing's syndrome. Clin Exp Hypertens 1995;17:1173-82. [Crossref] [PubMed]
- Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. J Clin Endocrinol Metab 2003;88:2384-92. [Crossref] [PubMed]
- Klomjit N, Rowan DJ, Kattah AG, et al. New-onset resistant hypertension in a newly diagnosed prostate cancer patient. Am J Hypertens 2019;32:1214-7. [Crossref] [PubMed]
- Stewart PM, Walker BR, Holder G, et al. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing's syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab 1995;80:3617-20. [PubMed]
- Yasuda G, Shionoiri H, Umemura S, et al. Exaggerated blood pressure response to angiotensin II in patients with Cushing's syndrome due to adrenocortical adenoma. Eur J Endocrinol 1994;131:582-8. [Crossref] [PubMed]
- Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A 1990;87:10043-7. [Crossref] [PubMed]
- Hirata F. Lipomodulin: a possible mediator of the action of glucocorticoids. Adv Prostaglandin Thromboxane Leukot Res 1983;11:73-8. [PubMed]
- Bönner G, Autenrieth R, Marin-Grez M, et al. Effects of sodium loading, desoxycorticosterone acetate, and corticosterone on urinary kallikrein excretion. Horm Res 1981;14:87-94. [Crossref] [PubMed]
- Hassan-Smith ZK, Sherlock M, Reulen RC, et al. Outcome of Cushing's disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab 2012;97:1194-201. [Crossref] [PubMed]
- Chow JT, Thompson GB, Grant CS, et al. Bilateral laparoscopic adrenalectomy for corticotrophin-dependent Cushing's syndrome: a review of the Mayo Clinic experience. Clin Endocrinol (Oxf) 2008;68:513-9. [Crossref] [PubMed]
- Patrova J, Kjellman M, Wahrenberg H, et al. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine 2017;58:267-75. [Crossref] [PubMed]
- Friedman JE, Yun JS, Patel YM, et al. Glucocorticoids regulate the induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem 1993;268:12952-7. [PubMed]
- Olefsky JM. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest 1975;56:1499-508. [Crossref] [PubMed]
- Wang CN, McLeod RS, Yao Z, et al. Effects of dexamethasone on the synthesis, degradation, and secretion of apolipoprotein B in cultured rat hepatocytes. Arterioscler Thromb Vasc Biol 1995;15:1481-91. [Crossref] [PubMed]
- Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 1997;99:414-23. [Crossref] [PubMed]
- Hollingdal M, Juhl CB, Dall R, et al. Glucocorticoid induced insulin resistance impairs basal but not glucose entrained high-frequency insulin pulsatility in humans. Diabetologia 2002;45:49-55. [Crossref] [PubMed]
- Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? Lancet 1997;349:1210-3. [Crossref] [PubMed]
- Faggiano A, Pivonello R, Spiezia S, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing's disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 2003;88:2527-33. [Crossref] [PubMed]
- Bancos I, Alahdab F, Crowley RK, et al. THERAPY OF ENDOCRINE DISEASE: Improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing's syndrome: a systematic review and meta-analysis. Eur J Endocrinol 2016;175:R283-95. [Crossref] [PubMed]
- Xu C, He J, Jiang H, et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol 2009;23:1161-70. [Crossref] [PubMed]
- Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496-506. [Crossref] [PubMed]
- Paterson JM, Morton NM, Fievet C, et al. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci U S A 2004;101:7088-93. [Crossref] [PubMed]
- Lemke U, Krones-Herzig A, Berriel Diaz M, et al. The glucocorticoid receptor controls hepatic dyslipidemia through Hes1. Cell Metab 2008;8:212-23. [Crossref] [PubMed]
- Rockall AG, Sohaib SA, Evans D, et al. Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol 2003;149:543-8. [Crossref] [PubMed]
- Muiesan ML, Lupia M, Salvetti M, et al. Left ventricular structural and functional characteristics in Cushing's syndrome. J Am Coll Cardiol 2003;41:2275-9. [Crossref] [PubMed]
- Pereira AM, Delgado V, Romijn JA, et al. Cardiac dysfunction is reversed upon successful treatment of Cushing's syndrome. Eur J Endocrinol 2010;162:331-40. [Crossref] [PubMed]
- Kamenický P, Redheuil A, Roux C, et al. Cardiac structure and function in Cushing's syndrome: a cardiac magnetic resonance imaging study. J Clin Endocrinol Metab 2014;99:E2144-53. [Crossref] [PubMed]
- Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med 1992;120:893-901. [PubMed]
- Ainscough JF, Drinkhill MJ, Sedo A, et al. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 2009;81:592-600. [Crossref] [PubMed]
- Albiger N, Testa RM, Almoto B, et al. Patients with Cushing's syndrome have increased intimal media thickness at different vascular levels: comparison with a population matched for similar cardiovascular risk factors. Horm Metab Res 2006;38:405-10. [Crossref] [PubMed]
- Vaidya A, Hamrahian A, Bancos I, et al. The Evaluation of Incidentally Discovered Adrenal Masses. Endocr Pract 2019;25:178-92. [Crossref] [PubMed]
- van der Pas R, de Herder WW, Hofland LJ, et al. New developments in the medical treatment of Cushing's syndrome. Endocr Relat Cancer 2012;19:R205-23. [Crossref] [PubMed]
- Gómez RM, Albiger NM, Diaz AG, et al. Effect of hypercortisolism control on high blood pressure in Cushing's syndrome. Medicina (B Aires) 2007;67:439-44. [PubMed]
- Sippel RS, Elaraj DM, Kebebew E, et al. Waiting for change: symptom resolution after adrenalectomy for Cushing's syndrome. Surgery 2008;144:1054-60; discussion 60-1. [Crossref] [PubMed]
- Alesina PF, Hommeltenberg S, Meier B, et al. Posterior retroperitoneoscopic adrenalectomy for clinical and subclinical Cushing's syndrome. World J Surg 2010;34:1391-7. [Crossref] [PubMed]
- Ntali G, Asimakopoulou A, Siamatras T, et al. Mortality in Cushing's syndrome: systematic analysis of a large series with prolonged follow-up. Eur J Endocrinol 2013;169:715-23. [Crossref] [PubMed]
- Sonino N, Boscaro M, Paoletta A, et al. Ketoconazole treatment in Cushing's syndrome: experience in 34 patients. Clin Endocrinol (Oxf) 1991;35:347-52. [Crossref] [PubMed]
- Moncet D, Morando DJ, Pitoia F, et al. Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing's syndrome. Medicina (B Aires) 2007;67:26-31. [PubMed]
- Castinetti F, Guignat L, Giraud P, et al. Ketoconazole in Cushing's disease: is it worth a try? J Clin Endocrinol Metab 2014;99:1623-30. [Crossref] [PubMed]
- Verhelst JA, Trainer PJ, Howlett TA, et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol (Oxf) 1991;35:169-78. [Crossref] [PubMed]
- Donadille B, Groussin L, Waintrop C, et al. Management of Cushing's syndrome due to ectopic adrenocorticotropin secretion with 1,ortho-1, para'-dichloro-diphenyl-dichloro-ethane: findings in 23 patients from a single center. J Clin Endocrinol Metab 2010;95:537-44. [Crossref] [PubMed]
- Fleseriu M, Biller BM, Findling JW, et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing's syndrome. J Clin Endocrinol Metab 2012;97:2039-49. [Crossref] [PubMed]
- Colao A, Petersenn S, Newell-Price J, et al. A 12-month phase 3 study of pasireotide in Cushing's disease. N Engl J Med 2012;366:914-24. [Crossref] [PubMed]
- Bertagna X, Pivonello R, Fleseriu M, et al. LCI699, a potent 11beta-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab 2014;99:1375-83. [Crossref] [PubMed]
- Fleseriu M, Pivonello R, Young J, et al. Osilodrostat, a potent oral 11beta-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing's disease. Pituitary 2016;19:138-48. [Crossref] [PubMed]
- Rizzoni D, Paiardi S, Rodella L, et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with primary aldosteronism. J Clin Endocrinol Metab 2006;91:2638-42. [Crossref] [PubMed]
- Toja PM, Branzi G, Ciambellotti F, et al. Clinical relevance of cardiac structure and function abnormalities in patients with Cushing's syndrome before and after cure. Clin Endocrinol (Oxf) 2012;76:332-8. [Crossref] [PubMed]
- Colao A, Pivonello R, Spiezia S, et al. Persistence of increased cardiovascular risk in patients with Cushing's disease after five years of successful cure. J Clin Endocrinol Metab 1999;84:2664-72. [PubMed]
- Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf) 1994;40:479-84. [Crossref] [PubMed]
- Preda VA, Sen J, Karavitaki N, et al. Etomidate in the management of hypercortisolaemia in Cushing's syndrome: a review. Eur J Endocrinol 2012;167:137-43. [Crossref] [PubMed]
- Feelders RA, de Bruin C, Pereira AM, et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing's disease. N Engl J Med 2010;362:1846-8. [Crossref] [PubMed]
- Castinetti F, Morange I, Jaquet P, et al. Ketoconazole revisited: a preoperative or postoperative treatment in Cushing's disease. Eur J Endocrinol 2008;158:91-9. [Crossref] [PubMed]
- Jeffcoate WJ, Rees LH, Tomlin S, et al. Metyrapone in long-term management of Cushing's disease. Br Med J 1977;2:215-7. [Crossref] [PubMed]
- Pivonello R, De Martino MC, Cappabianca P, et al. The medical treatment of Cushing's disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 2009;94:223-30. [Crossref] [PubMed]
- Webb SM, Mo D, Lamberts SW, et al. Metabolic, cardiovascular, and cerebrovascular outcomes in growth hormone-deficient subjects with previous cushing's disease or non-functioning pituitary adenoma. J Clin Endocrinol Metab 2010;95:630-8. [Crossref] [PubMed]
- Barahona MJ, Resmini E, Vilades D, et al. Coronary artery disease detected by multislice computed tomography in patients after long-term cure of Cushing's syndrome. J Clin Endocrinol Metab 2013;98:1093-9. [Crossref] [PubMed]
- van Haalen FM, Broersen LH, Jorgensen JO, et al. Management of endocrine disease: Mortality remains increased in Cushing's disease despite biochemical remission: a systematic review and meta-analysis. Eur J Endocrinol 2015;172:R143-9. [Crossref] [PubMed]
- Ragnarsson O, Olsson DS, Papakokkinou E, et al. Overall and Disease-Specific Mortality in Patients With Cushing Disease: A Swedish Nationwide Study. J Clin Endocrinol Metab 2019;104:2375-84. [Crossref] [PubMed]
- Graversen D, Vestergaard P, Stochholm K, et al. Mortality in Cushing's syndrome: a systematic review and meta-analysis. Eur J Intern Med 2012;23:278-82. [Crossref] [PubMed]
- Yaneva M, Kalinov K, Zacharieva S. Mortality in Cushing's syndrome: data from 386 patients from a single tertiary referral center. Eur J Endocrinol 2013;169:621-7. [Crossref] [PubMed]
- Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab 2006;91:371-7. [Crossref] [PubMed]
- Shah N, Ruiz HH, Zafar U, et al. Proinflammatory cytokines remain elevated despite long-term remission in Cushing's disease: a prospective study. Clin Endocrinol (Oxf) 2017;86:68-74. [Crossref] [PubMed]


