
Evaluation of surgical risk and prognosis between thyroid nodules of size <1 and ≥1 cm
Introduction
As the American Thyroid Association (ATA) guidelines in 2015 indicated: generally, only nodules >1 cm should be evaluated, because they have a greater potential to be clinically significant cancers. Occasionally, nodules <1 cm might require evaluation because of suspicious findings, associated lymphadenopathy, or other high-risk clinical factors such as a history of childhood head and neck irradiation or a history of thyroid carcinoma in one or more first-degree relatives (1). Therefore, the treatment for thyroid nodules <1 cm now is more inclined to the wait-and-see principle. As the unbalanced distribution of medical resources in China and many patients in economically underdeveloped regions have difficulty to adhering to long-term and regular follow-up (2), we wonder whether the wait-and-see strategy for thyroid nodule <1 cm applies for wide areas in China. Therefore, we retrospectively collected the data of 6,317 cases of first-time thyroid surgery in the past 12 years at Harbin Medical University and analyzed the surgical risk and prognosis between thyroid nodules <1 and ≥1 cm. Based on the research data and our clinical experiences, this study discusses whether the wait-and-see principle was an effective, safe, and appropriate procedure to manage thyroid diseases and provides insight into the management of thyroid diseases in wide areas of China.
Methods
Patients
A retrospective review was conducted of all patients older than 18 years who underwent a first-time thyroid surgery between January 2005 and December 2016 at The First Affiliated Hospital of Harbin Medical University. All clinical and pathological data was entered retrospectively in a computerized database. We extracted data include sex, age, radiological findings, laboratory results, surgical procedures, final pathological reports, and postoperative complications. The Medical Ethics Committee at The First Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang, China) approved this study (ID 2018014).
All operations were performed by the same skilled thyroid chief surgeon to diminish surgical deviation. Medical records that listed simultaneous diseases in the neck, such as hyperthyroidism, lymphoma, thyrohyoid cyst, and tuberculosis were excluded.
Preoperative evaluation
All patients underwent thyroid and cervical lymph node ultrasonography to obtain the nodule’s size, number, and location of to assess the characteristics and whether lymph node metastasis (LNM) had developed. Computed tomography (CT) was also performed on all patients to provide important adjunct anatomical information about the thyroid as well as related structures in the neck; it was a necessary preoperative imaging study that affected both the extent of surgery and the method of anesthesia induction (3,4). Patients underwent indirect laryngoscope examination before surgery to determine whether there was superior laryngeal nerve (SLN) and/or recurrent laryngeal nerve (RLN) injury before operation. Furthermore, we evaluated all patients’ thyroid stimulating hormone (TSH), thyroid peroxidase antibody (TPO-Ab), thyroglobulin antibody (Tg-Ab), and parathyroid hormone (PTH) before surgery to determine whether there was hyperthyroidism, Hashimoto’s thyroiditis, or parathyroid dysfunction. Given the objective medical conditions in China and the fact that the study involved patients 10 years ago, we did not require every patient of our study to complete fine-needle aspiration cytology (FNAC) examination.
Surgical approach
All patients underwent first-time thyroid surgery. According to the preoperative evaluation as mentioned, combining the results of intraoperative frozen section (IFS), the patients were treated with hemithyroidectomy (HT), total thyroidectomy (TT), TT and central lymph node dissection (TT + CLND), or TT and modified lymph node dissection (TT + MLND). The parathyroid gland was carefully removed and transplanted in the ipsilateral sternocleidomastoid muscle when preservation in its original position was difficult to achieve. Because parathyroid autograft (PAT) is an effective method to avoid long-term hypoparathyroidism (5), it was generally implemented in our center. Other irregular procedures were performed based on the specific findings during procedure.
Postoperative management and follow-up
The patients were prescribed a daily dose of 100–200 µg of levothyroxine replacement, according to their age, weight, and sex, and dynamically monitored TSH to adjust the dose. Daily oral intake of calcium and vitamin D supplementation (Caltrate D, Wyeth, USA, containing 1.5 g calcium carbonate and vitamin D3 125 IU in each tablet) for 2 weeks to all patients who had PAT, which was regarded as a safe and cost-effective method to prevent symptomatic hypocalcemia (6,7). If calcium supplements were still needed after 6 months, the parathyroid dysfunction was considered permanent. If the patient had symptoms such as hoarseness, low pitch of voice or drink-related cough, additional indirect laryngoscopy was scheduled at first, third, and 6th month after surgery or until the symptoms disappear. If it did not recover after 6 months, we considered SLN and/or RLN injury as permanent. Regularly review of thyroglobulin (Tg) for postoperative cancer to detect recurrent disease.
Follow-up data was obtained through telephone survey. The duration of calcium replacement time and accompanying symptoms (hoarseness, low pitch and drink-related cough) were assessed to determine whether hypoparathyroidism, SLN and/or RLN injury existed. Whether a patient suffered regional lymph node recurrence, distant metastasis or even disease-related death were ascertained to assess the patient's disease survival status. The follow-up time was up to May 2018.
Statistical analyses
Statistical analyses were conducted using SPSS for Windows (SPSS Statistics 24, IBM; Armonk, NY, USA). Qualitative variables were described by frequencies, and quantitative variables were described by their means and/or medians with variances and/or range. Student’s t-test was used to compare quantitative variables and chi-square for qualitative variables. The DFS curves of patients with thyroid carcinoma were complemented by GraphPad Prism 6.0 software. P<0.05 was considered statistically significant.
Results
General clinical data
A total of 6,317 first-time thyroid operations were performed between January 2005 and December 2016, 974 men and 5,343 women with a mean age of 51 (range, 19–80) years were included. According to the maximum diameter of nodules indicated by preoperative thyroid ultrasonography, all patients were divided into two groups: A: <1 cm (2,128, 33.69%); B: ≥1 cm (4,189, 66.31%). The two groups’ preoperative clinical data was analyzed and presented in Table 1. There were no significant differences between preoperative TSH level (P=0.094). Patients in group A presented younger average age (47.4±9.9 vs. 51.2±10.5, P=0.000), lower males’ rate (9.40% vs. 18.48%, P=0.000), fewer abnormal preoperative laryngoscopes (0.85% vs. 1.60%, P=0.014), fewer preoperative tracheal deviations (1.60% vs. 13.32%, P=0.000) and fewer suspicious LNM (11.09% vs. 9.48%, P=0.043). Moreover, patients in group A had fewer clinical symptoms as pain, hoarseness, and dyspnea at admission (2.40% vs. 8.88%, P=0.000; 1.36% vs. 9.07%, P=0.000; 10.71% vs. 16.78%, P=0.000, respectively).
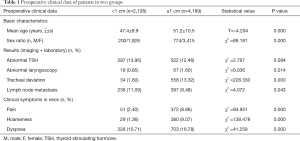
Full table
Postoperative pathological reports and surgical methods
According to IFS and postoperative routine pathological results, 2,893 benign thyroid diseases were diagnosed. Among the 3,424 malignant patients, 3,298 (96.32%) papillary thyroid carcinoma (PTC), 61 (1.78%) follicular thyroid carcinoma (FTC), 51 (1.49%) medullary thyroid carcinoma (MTC) and 14 anaplastic thyroid carcinoma (ATC) were diagnosed. FTC and PTC were defined as differentiated thyroid cancer (DTC), which normally has a better prognosis. MTC was associated with a high probability of metastasis was interposed between ATC and DTC (8). The pathological diagnoses of the two groups were analyzed and established that patients in group A had significantly more manageable thyroid disease (A vs. B, P=0.000) (Table 2), suggested that patients with thyroid nodules <1 cm had better prognosis.
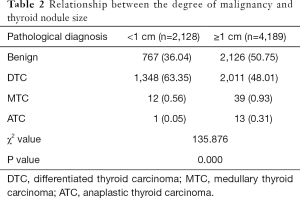
Full table
Among 3,424 malignant patients, 1,316 (38.43%) patients had varying degrees of LNM. Patients with nodules <1 cm presented a significantly lower rate and inferior extent of LNM (A vs. B, P=0.000) (Table 3), which suggests thyroid carcinoma with nodules <1 cm augurs less invasive behavior.
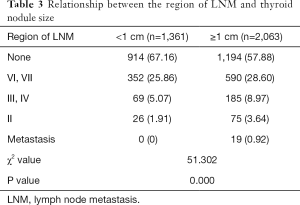
Full table
Of all patients, 1,019 (16.13%) underwent HT, 4,579 (72.49%) underwent TT, 539 (8.53%) underwent TT + CLND, and 140 (2.22%) underwent TT + MLND. In addition, according to the results of intraoperative exploration, 14 patients underwent RLN repair procedure, 6 patients underwent internal jugular vein tumor thrombus removal, 4 patients underwent a partial tracheal resection and repair procedure, 13 patients underwent tracheal suspension operation, and 3 patients underwent RLN resection and esophageal repair. Comparing the surgical procedures of the two groups, we found that patients with thyroid nodules <1 cm required significantly less range and complexity of surgery (A vs. B, P=0.000) (Table 4).
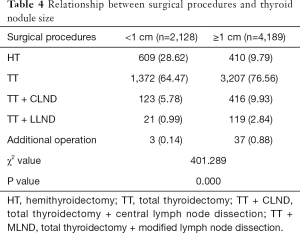
Full table
Postoperative complications and prognosis
By May 2018, the median follow-up time was 75 (range, 17–160) months, and only 4,565 (72.27%) patients were successfully followed. According to the follow-up results, 625 patients (596 transient, 29 permanent) with hypoparathyroidism were found, 290 patients (254 transient, 36 permanent) had SLN and/or RLN injury. We analyzed the postoperative complications of the two groups and found that patients with thyroid nodules <1 cm had statistically less postoperative hypoparathyroidism (A vs. B, P=0.008) but similar SLN and/or RLN injury (A vs. B, P=0.260) (Table 5).

Full table
A total of 2,278 (49.90%) patients with thyroid carcinoma followed up successfully. Up until the end of follow-up, 80 (3.51%) developed local lymph node recurrence, 21 (0.92%) developed distant metastasis, and 24 (1.05%) died from thyroid carcinoma. As the minor mortality and morbidity, the DFS of the two groups were analyzed and demonstrated in Figure 1, which suggests that patients with thyroid cancer <1 cm had a significantly longer DFS time (A vs. B, P=0.000).
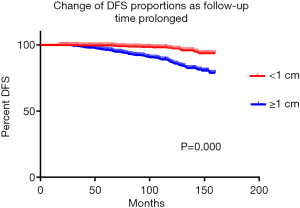
Together, these results indicate that early surgery, instead of a wait-and-see strategy, can not only reduce postoperative complications but also improve patients’ long-term survival.
Discussion
The reasons of increased thyroid diseases
Ahn et al. expressed concern about overdiagnosis of thyroid nodules and suggesting restraint in diagnosing and treating thyroid nodules <1 cm (9-12). They believed that advanced ultrasonography and people’s improved health awareness caused the growing incidence of thyroid carcinoma as the increasing cases of thyroid carcinoma and the stable mortality rate. However, a study in the United States reported the incidence of larger tumors has also increased, suggested that the increasing thyroid carcinoma may also be related to other factors (13). Multiple studies have shown that the rise in body mass index (BMI) is a risk factor (14-16). Moreover, the environment change (radiation exposure) (17) and dietary habit (excessive iodine intake) (18) are also associated with increased incidence of thyroid carcinoma.
Diagnosis approach
The thyroid imaging reporting and data system (TIRADS) is a new, powerful diagnostic technique that was recently applied in the diagnostic of nodular thyroid disease, however, the interpretation is related to the experience of the ultrasound physician. Nowadays, FNAC is the most efficient tool to sort which nodules should be referred for surgery, but cytological results remain indeterminate in 17–23% (19,20). Cavallo et al. (21) advocated that nodules of all sizes should undergo fine-needle aspiration (FNA) biopsy appropriate to guide further management. Facing with indeterminate cytological results, a return to TIRADS score is also of limited value in most conditions (22,23) Other options like repeated FNAC, iodine 123 scintigraphy, core needle biopsy, immunocytochemistry, and molecular testing can be valuable to increase diagnostic accuracy but not possibly available in all regions, and the cost-effectiveness is disputable (24,25).
Small nodules do not mean less invasive
In our study, 63.96% of thyroid nodules <1 cm were diagnosed as malignant, whereas only 49.25% of thyroid nodules ≥1 cm were diagnosed as malignant. The higher malignancy rate in thyroid nodules <1 cm may be the consequence of selection bias as the data came from the inpatients who usually required surgery, however, the high incidence of carcinoma in thyroid nodules <1 cm should not be ignored. Moreover, LNM rate was 32.85% in our study consistent with the results of other published studies ranging from 20% to 66% (26-29). Though LNM may not threat the survival, it is associated with a higher risk of recurrence (30), and then affect the patient’s quality of long-term life.
Papillary thyroid microcarcinoma (PTMC) was widely believed to offer a good prognosis, but several studies reported that PTMC may cause distant metastasis (31-36), and 0.34–1% of patients may die from PTMC (37-42). Therefore, size alone should not be a predictor of malignancy, and PTMC does not mean low-risk disease.
Moreover, several studies have demonstrated that ATC may derive from DTC in certain cases: RAS mutations are thought to initiate the dedifferentiation process from DTC to ATC (43); p53 is one of the genetic events distinguishing ATC from poorly differentiated thyroid carcinoma (PDTC) (44). BRAF mutations are a seminal molecular event that triggers thyroid carcinogenesis or dedifferentiation or confers aggressiveness (45). In addition, dedifferentiation would increase along with tumor survival and aging. As the current inability of diagnostic accurately, the heterogeneity of PTMC cannot be ignored. Once diagnosed, the treatment should be performed the same as PTC: based on HT and lymph node dissection should be performed according to the specific case to help improve survival from this treatable disease.
Safety of thyroid surgery
Thyroid has a close relationship with parathyroid glands, SLNs, RLNs, esophagus, trachea, and common carotid artery and vein. These structures play important roles in normal physiological functions and injure to them may be harmful to the patient’s quality of life that must be safeguarded during thyroid operations. Increasing thyroid nodules may cause more difficulties in surgery and lead to more complications. The author often incidentally observed an inflammatory or cancerous lesion closely connected to a parathyroid gland and/or RLN. When the lesion is tiny, we may take advantage of careful surgery to ensure the integrity of the structures and simultaneously perform radical resection, but if we continue to wait-and-see as the lesion increases, the feasibility and safety of surgery may be reduced just as the results of our study (Table 5), patients with lesions ≥1 cm suffered more and heavier postoperative hypoparathyroidism than those with <1 cm.
ATA guidelines in China
When the ATA guidelines are applied to China, we need to consider the specific conditions there. First, as China’s huge population base, even with an extremely low mortality rate, 6,800 patients died of thyroid carcinoma in 2015, which is alarming (46). Second, studies have shown that the 5-year relative survival rate of thyroid carcinoma patients is 98.2% in the United States (47); 86.5% in Europe (48); but only 67.5% in China (49). Moreover, unlike the stable mortality in developed countries such as Europe and the United States, the mortality rate of thyroid carcinoma in China is rising at a rate of 1.4% per year (50). Third, as some hospitals in developed areas of China offer similar postoperative prognoses to those in the United States and Europe that the distribution of medical resources in China is uneven (2,51). Due to the unbalance and personal economic problems, many patients have difficulties adhering to long-term and regular follow-up, especially in economically underdeveloped areas. If the proper treatment is not carried out in time, the disease may progress without monitoring; meanwhile, most of the patients cannot bear the pressure of treating advanced and recurrent carcinoma either psychologically or economically. Finally, as the increasingly tense situation of contradictions between doctors and patients in China, if the diagnosis of high-risk thyroid carcinoma is neglected and the treatment is delayed, there may emerge medical disputes. Therefore, the principle of wait-and-see for general thyroid nodules <1 cm and conservative treatment for PTMC may not be universally applicable in wide areas of China.
Considering these reasons, patients with thyroid nodules in China should be given the best medical care based on evidence and the specific conditions of the patients. In economically developed areas, the patient can be treated according to the ATA guidelines if he or she is in good compliance, with strong nonsurgical willingness, and has been fully informed about the disease risk. However, for patients in less-developed areas, with poor compliance, early surgical treatment is recommended for suspicious carcinoma to obtain a better prognosis, fewer complications, and less psychological and financial burden. In addition, China should strengthen the training of professional thyroid surgeons and improve abilities to diagnose and treat thyroid diseases to benefit more patients.
Limitations
As this study involves some patients more than 10 years ago, several advanced examinations like TIRADS and FNAC cannot be observed. As a retrospective study; the subjects were all inpatients who tended to need surgery, and there may be patients with advanced cancer and high surgical risk who abandoned treatment. Therefore, there might be selection bias. In addition, due to the long telephone follow-up period, the rate of loss was high, and the results may have recall bias and loss bias. Therefore, large-sample, multicenter, prospective, randomized controlled trials are still needed to validate the results of this study further.
Conclusions
Thyroid operations for thyroid nodules <1 cm have fewer postoperative complications than nodules ≥1 cm. Patients who were diagnosed with malignant thyroid disease had better prognoses with nodules <1 cm. Underdeveloped regions of China should not ignore the treatment and diagnose of patients with thyroid nodules <1 cm.
Moreover, thyroid carcinoma is malignant disease and treatment should follow the same principles as other malignant tumors: earlier detection, diagnosis, and treatment. Abandoning monitoring a malignant disease because of its overall good prognosis violates ethical principles; deterring examination an increasing lesion on account of the high medical burden breaches medical science.
Acknowledgments
Funding: This study was funded by the National Nature Scientific Foundation of China (No. 81770639).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Medical Ethics Committee at The First Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang, China) approved this study (ID 2018014).
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Tian W, Xi HQ. Analysis of the survival status of patients with thyroid carcinoma. Chinese Journal of Practical Surgery 2016;36:489-93.
- Li L, Wang Y, Zhao Y, et al. Evaluation with low-dose dual-phase helical computed tomography of patients with thyroid lesions. Chin Med J (Engl) 2014;127:3937-43. [PubMed]
- Han ZJ, Shu YY, Lai XF, et al. Value of computed tomography in determining the nature of papillary thyroid microcarcinomas: evaluation of the computed tomographic characteristics. Clin Imaging 2013;37:664-8. [Crossref] [PubMed]
- Palazzo FF, Sywak MS, Sidhu SB, et al. Parathyroid autotransplantation during total thyroidectomy--does the number of glands transplanted affect outcome? World J Surg 2005;29:629-31. [Crossref] [PubMed]
- Abboud B, Sleilaty G, Zeineddine S, et al. Is therapy with calcium and vitamin D and parathyroid autotransplantation useful in total thyroidectomy for preventing hypocalcemia? Head Neck 2008;30:1148-54; discussion 1154-5. [Crossref] [PubMed]
- Singer MC, Bhakta D, Seybt MW, et al. Calcium management after thyroidectomy: a simple and cost-effective method. Otolaryngol Head Neck Surg 2012;146:362-5. [Crossref] [PubMed]
- Dackiw AP. The surgical management of medullary thyroid cancer. Otolaryngol Clin North Am 2010;43:365-74. ix. [Crossref] [PubMed]
- Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med 2014;371:1765-7. [Crossref] [PubMed]
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 2016;375:614-7. [Crossref] [PubMed]
- Hoang JK, Nguyen XV. Understanding the risks and harms of management of incidental thyroid nodules: a review. JAMA Otolaryngol Head Neck Surg 2017;143:718-24. [Crossref] [PubMed]
- Kim SY, Lee HS, Kim EK, et al. Follow-up ultrasound may be enough for thyroid nodules from 5 mm to 1 cm in size. Endocrine 2016;52:130-8. [Crossref] [PubMed]
- Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in The United States, 1988-2005. Cancer 2009;115:3801-7. [Crossref] [PubMed]
- Clavel-Chapelon F, Guillas G, Tondeur L, et al. Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. Int J Cancer 2010;126:2984-90. [PubMed]
- Leitzmann MF, Brenner A, Moore SC, et al. Prospective study of body mass inde, physical activity and thyroid cancer. Int J Cancer 2010;126:2947-56. [PubMed]
- Meinhold CL, Ron E, Schonfeld SJ, et al. Nonradiation risk factors for thyroid cancer in the US radiologic technologists study. Am J Epidemiol 2010;171:242-52. [Crossref] [PubMed]
- WHO/IARC. World Cancer Report 2014. Lyon: IARC Press, 2014:738-50.
- Lee JH, Hwang Y, Song RY, et al. Relationship between iodine levels and papillary thyroid carcinoma: a systematic review and meta-analysis. Head Neck 2017;39:1711-8. [Crossref] [PubMed]
- Russ G, Royer B, Bigorgne C, et al. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol 2013;168:649-55. [Crossref] [PubMed]
- Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol 2012;56:333-9. [Crossref] [PubMed]
- Cavallo A, Johnson DN, White MG, et al. Thyroid nodule size at ultrasound as a predictor of malignancy and final pathologic size. Thyroid 2017;27:641-50. [Crossref] [PubMed]
- Yang BR, Kim EK, Moon HJ, et al. Qualitative and semiquantitative elastography for the diagnosis of intermediate suspicious thyroid nodules based on the 2015 American Thyroid Association Guidelines. J Ultrasound Med 2018;37:1007-14. [Crossref] [PubMed]
- Chaigneau E, Russ G, Royer B, et al. TIRADS score is of limited clinical value for risk stratification of indeterminate cytological results. Eur J Endocrinol 2018;179:13-20. [Crossref] [PubMed]
- Suh CH, Baek JH, Park C, et al. The role of core needle biopsy for thyroid nodules with initially indeterminate results on previous fine-needle aspiration: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017;38:1421-6. [Crossref] [PubMed]
- Hahn SY, Shin JH, Lim HK, et al. Follicular variant of papillary thyroid carcinoma: comparison of ultrasound-guided core needle biopsy and ultrasound-guided fine needle aspiration in a multicentre study. Clin Endocrinol (Oxf) 2017;86:113-9. [Crossref] [PubMed]
- Zhao Q, Ming J, Liu C, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol 2013;20:746-52. [Crossref] [PubMed]
- Lim YC, Choi EC, Yoon YH, et al. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg 2009;96:253-7. [Crossref] [PubMed]
- Kim KE, Kim EK, Yoon JH, et al. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg 2013;37:385-91. [Crossref] [PubMed]
- Vergez S, Sarini J, Percodani J, et al. Lymph node management in clinically node-negative patients with papillary thyroid carcinoma. Eur J Surg Oncol 2010;36:777-82. [Crossref] [PubMed]
- Domínguez JM, Nilo F, Martínez MT, et al. Papillary Thyroid Microcarcinoma: Characteristics at presentation, and evaluation of clinical and histological features associated with a worse prognosis in a latin american cohort. Arch Endocrinol Metab 2018;62:6-13. [Crossref] [PubMed]
- Ito Y, Uruno T, Nakano K, et al. An Observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [Crossref] [PubMed]
- Pellegriti G, Scollo C, Lumera G, et al. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: Study of 299 Cases. J Clin Endocrinol Metab 2004;89:3713-20. [Crossref] [PubMed]
- Baudin E, Travagli JP, Ropers J, et al. Microcarcinoma of the thyroid gland: the gustave-roussy institute experience. Cancer 1998;83:553-9. [Crossref] [PubMed]
- Sugitani I, Yanagisawa A, Shimizu A, et al. Clinicopathologic and immunohistochemical studies of papillary thyroid microcarcinoma presenting with cervical lymphadenopathy. World J Surg 1998;22:731-7. [Crossref] [PubMed]
- Appetecchia M, Scarcello G, Pucci E, et al. Outcome after treatment of papillary thyroid microcarcinoma. J Exp Clin Cancer Res 2002;21:159-64. [PubMed]
- Lo CY, Chan WF, Lang BH, et al. Papillary Microcarcinoma: is there any difference between clinically overt and occult tumors? World J Surg 2006;30:759-66. [Crossref] [PubMed]
- Yu XM, Wan Y, Sippel RS, et al. Should All Papillary Thyroid Microcarcinomas be aggressively treated? An Analysis of 18,445 Cases. Ann Surg 2011;254:653-60. [Crossref] [PubMed]
- Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer 2003;98:31-40. [Crossref] [PubMed]
- Roti E. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol 2008;159:659-73. [Crossref] [PubMed]
- Xu YH, Song HJ, Qiu ZL, et al. Brain metastases with exceptional features from papillary thyroid carcinoma: report of three cases. Hell J Nucl Med 2011;14:56-9. [PubMed]
- Ardito G, Revelli L, Giustozzi E, et al. Aggressive papillary thyroid microcarcinoma: prognostic factors and therapeutic strategy. Clin Nucl Med 2013;38:25-8. [Crossref] [PubMed]
- Cappelli C, Castellano M, Braga M, et al. Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol 2007;95:555-60. [Crossref] [PubMed]
- Howell GM, Hodak SP, Yip L. RAs mutations in thyroid cancer. Oncologist 2013;18:926-32. [Crossref] [PubMed]
- Xu B, Ghossein R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr Pathol 2016;27:205-12. [Crossref] [PubMed]
- Lim AM, Taylor GR, Fellowes A, et al. BRAF inhibition in BRAFV600E-positive anaplastic thyroid carcinoma. J Natl Compr Canc Netw 2016;14:249-54. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in china, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Thyroid cancer [OL]. [2015-06-17]. Available online: http://seer.cancer.gov/statfacts/html/thyro.html
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol 2014;15:23-34. [Crossref] [PubMed]
- Zeng H, Zheng R, Guo Y, et al. Cancer survival in china, 2003-2005: a population-based study. Int J Cancer 2015;136:1921-30. [Crossref] [PubMed]
- Liu YQ, Zhang SQ, Chen WQ, et al. Trend of incidence and mortality on thyroid cancer in China during 2003 - 2007. Zhonghua Liu Xing Bing Xue Za Zhi 2012;33:1044-8. [PubMed]
- Tang T, Tan HD, Huang LP. The clinical analysis of the factors that influence the prognosis of differentiated thyroid carcinoma (DTC) in the young-middle patients. China Prac Med 2007;2:1-4. [Crossref]


