
Intraoperative nerve monitoring in thyroid surgery—shifting current paradigms
Introduction
With the volume of thyroid operations expanding in recent years, so did risk minimization measures protecting the recurrent laryngeal nerve (RLN). Although the incidence of RLN palsy is low in experienced hands, postoperative compromise of voice quality, when permanent, may diminish the patient’s quality of life and trigger litigation for malpractice (1). Intraoperative neural monitoring (IONM), if properly performed and documented, offers some protection in a litigious environment. IONM comprises three interdependent steps of nerve evaluation: preoperative, intraoperative, and postoperative monitoring of RLN function, which add another dimension to thyroid surgery (2). Advanced surgical skills, including visual identification and dissection of the RLN along its anatomical course, are important prerequisites.
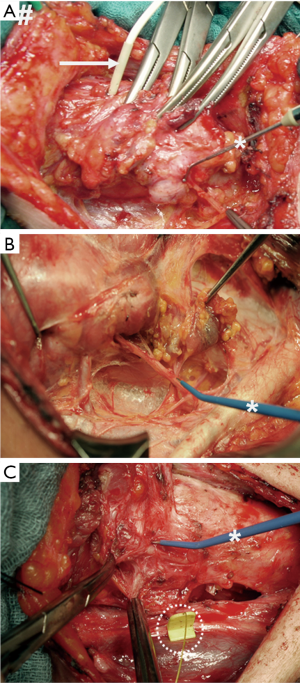
Figure 1 illustrates successive stages in the evolution of IONM:
- Intermittent nerve stimulation, with hand-held monopolar probes stimulating the RLN and needle electrodes inserted through the cricothyroid membrane into the vocal muscle recording the electrophysiological response signal (Figure 1A);
- Intermittent nerve stimulation, with hand-held monopolar probes stimulating the RLN and surface electrodes affixed to the orotracheal tube recording the electrophysiological response signal (Figure 1B);
- Continuous nerve stimulation, with a clip electrode mounted on the vagus nerve and surface electrodes affixed to the orotracheal tube recording the electrophysiological response signal (Figure 1C).
Although the use of IONM has gained increasing popularity and is considered a standard of care in some countries, the cost-effectiveness of this technology remains controversial in resource-conscious environments (3). The review summarizes the advances of continuous IONM technology that caused a quantum leap in risk minimization for thyroid surgery, shifting current paradigms.
Strengths and weaknesses of meta-analyses of intermittent IONM
Published systematic reviews and meta-analyses of studies comparing IONM against visual nerve identification in the absence of IONM are plentiful (3-12). Surprisingly, many of these systematic reviews and meta-analyses arrived at opposing conclusions. There was evidence to suggest that IONM decreases both transient and permanent RLN injury (4,12), only transient RLN injury (3,11), or only permanent RLN injury (10). By way of contrast, other researchers found no substantial reduction of permanent RLN injury (6), or both transient and permanent RLN injuries (7-9). These heterogeneous, at times conflicting results are best explained, at least in part, by the stepwise evolution of IONM technology and gradual recognition of the importance of standardization based on the principles laid down by the International Neural Monitoring Study Group (13). Almost never included in systematic reviews and meta-analyses were studies using continuous vagal IONM, the most recent and most advanced variety of IONM. This observation hints at incomplete adoption of this novel technology by national healthcare systems.
Best practice of IONM in thyroid surgery
Current nerve monitors, using intermittent or continuous IONM, facilitate RLN identification and mapping of the anatomic course of the nerve. Both intermittent and continuous IONM are able to differentiate between segmental loss of signal (LOS) type 1 and diffuse LOS type 2, whereas continuous IONM offers add-on benefits (Table 1). Both IONM modalities inform surgical treatment plan and provide strategic direction regarding the need for staged thyroidectomy in the event of definitive LOS on the first side of the operation (1). The German Association of Endocrine Surgeons (14) and the Australian College of Surgeons (15) both advocate the use of IONM as a valuable adjunct to RLN visualization.

Full table
It may also be worthy of note that there is a clinical utility gradient: from visual nerve identification in the absence of IONM to intermittent IONM and recently to continuous IONM (Table 1).
Many surgical disciplines apply IONM routinely. Because it can accurately predict postoperative vocal fold function, IONM has become a standard of care in some countries (16). Continuous IONM, the most recent addition to the armamentarium of IONM, provides for intraoperative registration of nerve signals virtually in real time. It has been demonstrated that instant release of a distressed nerve minimizes RLN injury. For documentation purposes, the electromyographic recordings can be printed out from the system and filed with the patient’s clinical chart.
Intraoperative prediction of postoperative vocal fold function
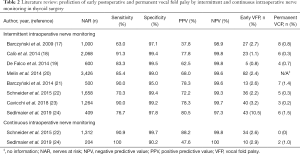
The performance of IONM for prediction of postoperative vocal fold palsy (Table 2), irrespective of frequency, is characterized by its:
- Sensitivity: 63.0–91.3% for intermittent, and 90.9–100% for continuous IONM; and
- Specificity: 97.1–99.5% for intermittent, and 90.2–99.7% for continuous IONM.
Full table
For rare outcomes, such as RLN palsy, the positive predictive value (PPV) is bound to be lower than the negative predictive value (NPV):
- PPV: 37.8–80.5% for intermittent, and 47.6–88.2% for continuous IONM; and
- NPV: 97.3–99.8% for intermittent, and 99.8–100% for continuous IONM;
These low rates of postoperative vocal fold palsy (Table 2);
- Early palsy: 0.8–10.5% for intermittent, and 2.6–2.9% for continuous IONM), and;
- Permanent palsy (0.2–1.5% for intermittent, and 0–1.0% for continuous IONM), attest to the high level of safety of thyroid operations in experienced hands.
To achieve these excellent outcomes, the International Neural Monitoring Study Group’s L1, V1, R1, R2, V2, L2 approach and troubleshooting algorithm for LOS must be followed (13). For optimal IONM results, it is important to obtain nerve amplitudes of ≥500 µV at baseline and use a stimulation current of 1 mA (25).
The key strength of continuous IONM, as compared to intermittent IONM, is seamless monitoring of the functional integrity along the entire vagus-RLN axis, virtually in real-time, for detection of impending traction-related RLN injury (25-29). In experienced hands, continuous IONM can diminish permanent vocal fold palsy rates to 0% (1,314 nerves at risk), comparing favourably against 0.4% with intermittent IONM (965 nerves at risk; P=0.019) (22).
Greater flexibility in thyroid mobilization and resection
Continuous IONM technique is a powerful tool for preventive and remedial action, enabling immediate recognition of RLN injury and correction of harmful surgical maneuvers. A large multicenter study with LOS for 115 nerves uncovered that traction produced 83% of RLN injuries, 60% of which took place near or at the ligament of Berry (30). These findings highlight the importance of IONM-guided thyroid mobilization and resection.
As a rule, traction-related injuries are preceded by so-called “combined events”, concordant changes in amplitude (≥50% decrease) and latency (>10% increase) relative to baseline. If not reacted on, these combined events may progress to LOS, a serious and less reversible condition (25,26,31). When such combined events happen, it is crucial to release the nerve immediately by relaxing one’s hold on the thyroid until the nerve amplitude has regained ≥50% of its baseline. If these so-called events reoccur more than once, it may be more prudent to embark on a median route to thyroid hilum than continuing a risky dissection laterally or from the upper or lower thyroid poles.
Continuous IONM is extremely beneficial in surgically demanding scenarios: recurrent disease fused with the thyroid capsule over a distance; thyroid tumor encroachment on adjacent organs, decompensated Graves’ disease, or extensive thyroid nodular disease involving the mediastinum and/or displacing the trachea. When the operative field is scarred and surgical layers are obliterated, continuous IONM can show its true colors: during mobilization of the thyroid, during dissection of the thyroid capsule off its bed, or during digital mobilization of retrosternal thyroid parts out of the mediastinum. In these situations, it is often impossible to identify and map the nerves. This is when continuous IONM comes into play. With the stimulating electrode mounted on the vagus nerve and activated, the operative team is constantly alerted of impending injury to a nerve that remains invisible (32). An unusual intraoperative finding is a RLN, grossly invaded by tumor, with normal vocal fold function on preoperative laryngoscopy. If grossly invaded nerves need to be preserved, dissecting those free from adjacent tumor is much easier with the support of continuous IONM.
Intraoperative amplitude recovery and postoperative vocal fold function
On receiver-operating characteristic analysis, relative and absolute amplitude recovery of 49% and 455 µV after segmental LOS type 1, and 44% or 253 µV after global LOS type 2 during the operation discriminated best between normal and abnormal early postoperative vocal fold function. In predicting early postoperative vocal fold function, absolute amplitude thresholds did not fare better after segmental LOS type 1 than relative amplitude thresholds, or fared even worse after global LOS type 2 (26,33). Pragmatically, the same unified relative amplitude threshold of ≥50% is used for segmental LOS type 1 and global LOS type 2.
After LOS, segmental type 1 nerve injury typically resolves within 6.9 to 8.0 minutes; and global type 2 injury within 13.0 to 15.6 minutes. This observation illustrates the futility of waiting longer than 20 minutes for the nerve to regain ≥50% of its baseline (26,33).
Intraoperative amplitude recovery of ≥50% relative to baseline after LOS accurately predicts normal early postoperative vocal fold function in all patients after transient segmental LOS type 1 or global LOS type 2. Early vocal fold function is impaired in 25% of patients with temporary global LOS type 2 and 64% of patients with temporary segmental LOS type 1 (33).
Same-session thyroidectomy versus staged thyroidectomy
When LOS has struck on the first side of resection and traction on the nerve has ceased immediately, it is worthwhile to give the nerve some 20 minutes to regain ≥50% of its preoperative baseline (Figure 2). If the nerve fails to do so within 20 minutes, there is a 85% risk of early postoperative vocal fold palsy (26). In that event, it is prudent to postpone completion of the other side for at least three months until vocal fold function has fully recovered (34,35). If vocal fold palsy persists for more than three months, the benefit-risk balance of contralateral completion surgery should be carefully revisited.
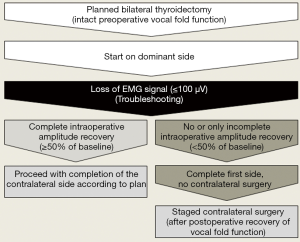
Only under exceptional circumstances, including advanced cancer, should contralateral surgery be pursued (36), and if so, solely at surgical centers experienced in complex neck surgery. Whenever the thyroid condition at hand is benign and not life-threatening, interests of patient safety should always take precedence over considerations of economy and convenience (37).
Because up to one-third of patients may not develop postoperative vocal fold palsy after what was interpreted as LOS on intermittent IONM, the concept of two-staged thyroidectomy has been questioned. A recent publication recommended one-stage contralateral thyroidectomy in the presence of persistent LOS on the first side to avoid the expense, psychological burden and potential complications of a second procedure (38).
A possible compromise, balancing the risk of recurrent disease with the need to stay away from the only functioning nerve, would be subtotal resection of the contralateral thyroid lobe in the same session. To protect the intact nerve, the line of resection must be kept ventral to the plane of the thyroid capsule at a distance from the RLN (36).
Current patterns of use of intermittent and continuous IONM
A recent German Society of Endocrine Surgeons (CAEK) survey, based on 12,888 patients with benign goiter and 18,793 nerves at risk registered on the German multicenter StudDoQ/Thyroid data base (39), found that IONM was used in 98.4% of patients, and that intermittent IONM (82.6%) was used more often than continuous IONM (17.4%). These results corroborated a 2012 questionnaire survey of German surgical centers which showed that IONM was employed in 91% of thyroidectomies (34). Within the past four 4 years, the percentage of continuous IONM use in Germany seems to have doubled (personal information).
In a 2016 survey of recently accredited fellows of the American Association of Endocrine Surgeons or American Head and Neck Society, 95% of respondents stated that they used IONM in 60% routinely and in 35% selectively. The most common line of reasoning for using IONM was increased surgeon confidence (55%) and improved safety (54%) (40). A 2019 survey of 1,015 surgeons certified by the American Academy of Otolaryngology-Head and Neck Surgery, the International Association of Endocrine Surgeons, or the American Head and Neck Society reported greater adoption of IONM for thyroid and parathyroid surgery in the past decade, with 83% of surgeons employing IONM for some or all of their operations. Uptake of IONM is greater among younger surgeons but is unrelated to surgical volume, fellowship training, or type of practice (41).
Honing surgical skills by constant feedback on nerve function
The initial phase of traction-related nerve injury is characterized by a reduction in the number of action potential-transmitting nerve fibers, causing the nerve amplitude to decrease. The next phase of injury is marked by progression of amplitude decrease and increasing new-onset latency, pointing to loss of myelin sheaths (27). Injury to a circumscript section of the nerve, giving rise to segmental type 1 injury, is produced by transection, clamping, ligation, pinching, or bipolar coagulation.
Continual electrophysiological feedback on the functional state of a nerve enables the surgical team to reflect on, and hone their surgical skills (1). This reassurance is likely to reduce stress, giving the surgical team a peace of mind (42). Employing IONM routinely in thyroid surgery also helps less experienced surgeons to perform operations more safely, with complication rates similar to those seen under supervision by an experienced surgeon (43).
Informed consent for and documentation of IONM
Ethical considerations demand the use of reasonable risk minimization strategies even if the expected benefits have not been clearly established (44). An informed consent detailing the strengths and weaknesses of IONM, including the need to change operative treatment plans in the event of LOS (45), may serve as a line of defense in the event of litigation (46). Many patients are eager to take an active role in shared decision-making, including consideration of intraoperative findings like LOS in the operative course of action (47).
In 2012, Dralle et al. (48) reported 75 lawsuits in a 15-year period for Germany, 60% of which involved RLN palsies, half of which were bilateral. At the time, IONM had been used in no more than six patients. Plaintiff verdicts based on omissions to identify the vagus nerve or due to neglect of LOS on the first side of resection in bilateral vocal fold palsy.
Gartland et al. (49) corroborated these findings after questioning the Controlled Risk Insurance Company Strategies’ Comparative Benchmarking System database. The authors found that bilateral RLN injury, accounting for up 18% of 128 malpractice suits in the US, was predictive of plaintiff verdicts (OR 3.58, P=0.03) on multivariable regression analysis.
These plaintiff verdicts underscore the importance of implementing, and properly documenting, the use of risk minimization strategies, notably vagus nerve identification and staged thyroidectomy. Documentation of normal vagus and RLN electrophysiology before (V1 and R1) and after (R2 and V2) dissection, printed out and filed with the patient’s chart, provides a strong line of defense against unfounded claims.
Cost-effectiveness of IONM technology
Reimbursement of a novel technology by third party payers is an important determinant of penetration of modern healthcare. Unsurprisingly, the uptake of IONM varies by country and geographic region. Costs also vary from $5,000 to $40,000 for the IONM equipment and from $72 to $500 for disposables (vagus electrodes and recording surface tubes) per application.
Rarely, if ever, included in these cost estimates are indirect costs, such as those for a Glidescope® video laryngoscope to ensure correct positioning of the recording tube electrodes, or the need for ancillary staff in the operating suite to aid in monitoring RLN function. Notoriously difficult to calculate are knock-on effects, such as costs incurred for treatment of vocal fold palsy or for unnecessary staged thyroidectomy prompted by false-positive IONM signals. The use of IONM may amount to 5–7% of the total inpatient expense charged for thyroidectomy (50).
Several studies questioned the cost-effectiveness of routine use of IONM for prevention of surgical complications, which are infrequent in community-based thyroid surgery (51). As the risk of operative complications increases, so does the potential for savings and hence the cost-effectiveness of IONM. When the percentage of RLN injury is reduced by ≥50.4% in comparison with visual nerve identification, IONM proved cost-effective (52). Economic modeling simulations confirmed that IONM is cost-effective in preventing permanent RLN palsy (53) and bilateral RLN paly based on quality-adjusted life-years (54).
At times, postoperative laryngoscopy to determine vocal fold function is not performed routinely in the absence of gross voice changes. Failure to consistently ascertain postoperative vocal fold function is bound to underestimate the true incidence of surgical vocal fold palsy (55).
Conclusions
Rigorous standardization and quality control managed to overcome those teething issues that continued to haunt IONM in the 1990s and 2000s. This technologic progress augmented the precision of IONM in signalling impending nerve injury early on and in correctly predicting postoperative vocal fold palsy (Table 2). This feedback loop enables surgical teams to reflect on, and hone their surgical skills.
Since the 2010s, IONM has matured into a powerful risk minimization instrument firmly integrated into thyroid surgery. As endoscopic and robotic surgery is gaining momentum in the Western hemisphere, so does IONM technology need to keep pace with these technological developments. There is a perennial need for adaptation of technology such as IONM to new uses (32,56). These advances caused a quantum leap in risk minimization for thyroid surgery, shifting current paradigms.
Acknowledgments
None.
Footnote
Conflicts of Interest: H Dralle was remunerated by Medtronic and Inomed for giving lectures on intraoperative nerve monitoring. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Smith J, Douglas J, Smith B, et al. Assessment of recurrent laryngeal nerve function during thyroid surgery. Ann R Coll Surg Engl 2014;96:130-5. [Crossref] [PubMed]
- Schneider R, Machens A, Randolph GW, et al. Opportunities and Challenges of Intermittent and Continuous Intraoperative Neural Monitoring in Thyroid Surgery. Gland Surg 2017;6:537-45. [Crossref] [PubMed]
- Yang S, Zhou L, Lu Z, et al. Systematic review with meta-analysis of intraoperative neuromonitoring during thyroidectomy. Int J Surg 2017;39:104-13. [Crossref] [PubMed]
- Bai B, Chen W. Protective Effects of Intraoperative Nerve Monitoring (IONM) for Recurrent Laryngeal Nerve Injury in Thyroidectomy: Meta-analysis. Sci Rep 2018;8:7761. [Crossref] [PubMed]
- Henry BM, Graves MJ, Vikse J, et al. The current state of intermittent intraoperative neural monitoring for prevention of recurrent laryngeal nerve injury during thyroidectomy: a PRISMA-compliant systematic review of overlapping meta-analyses. Langenbecks Arch Surg 2017;402:663-73. [Crossref] [PubMed]
- Lombardi CP, Carnassale G, Damiani G, et al. "The final countdown": Is intraoperative, intermittent neuromonitoring really useful in preventing permanent nerve palsy? Evidence from a meta-analysis. Surgery 2016;160:1693-706. [Crossref] [PubMed]
- Malik R, Linos D. Intraoperative Neuromonitoring in Thyroid Surgery: A Systematic Review. World J Surg 2016;40:2051-8. [Crossref] [PubMed]
- Pisanu A, Porceddu G, Podda M, et al. Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 2014;188:152-61. [Crossref] [PubMed]
- Sanabria A, Ramirez A, Kowalski LP, et al. Neuromonitoring in thyroidectomy: a meta-analysis of effectiveness from randomized controlled trials. Eur Arch Otorhinolaryngol 2013;270:2175-89. [Crossref] [PubMed]
- Sun W, Liu J, Zhang H, et al. A meta-analysis of intraoperative neuromonitoring of recurrent laryngeal nerve palsy during thyroid reoperations. Clin Endocrinol (Oxf) 2017;87:572-80. [Crossref] [PubMed]
- Wong KP, Mak KL, Wong CK, et al. Systematic review and meta-analysis on intra-operative neuro-monitoring in high-risk thyroidectomy. Int J Surg 2017;38:21-30. [Crossref] [PubMed]
- Zheng S, Xu Z, Wei Y, et al. Effect of intraoperative neuromonitoring on recurrent laryngeal nerve palsy rates after thyroid surgery--a meta-analysis. J Formos Med Assoc 2013;112:463-72. [Crossref] [PubMed]
- Randolph GW, Dralle H. International Neuromonitoring Stuy Group. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16. [Crossref] [PubMed]
- Dralle H, Lorenz K, Schabram P, et al. Intraoperative neuromonitoring in thyroid surgery. Recommendations of the Surgical Working Group for Endocrinology. Chirurg 2013;84:1049-56. [Crossref] [PubMed]
- Serpell J, Sidhu S, Vallance N, et al. Consensus statement on intra-operative electrophysiological recurrent laryngeal nerve monitoring during thyroid surgery. ANZ J Surg 2014;84:603-4. [Crossref] [PubMed]
- Hayward NJ, Grodski S, Yeung M, et al. Recurrent laryngeal nerve injury in thyroid surgery: a review. ANZ J Surg 2013;83:15-21. [Crossref] [PubMed]
- Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus nerve monitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009;96:240-6. [Crossref] [PubMed]
- Calò PG, Pisano G, Medas F, et al. Identification alone versus intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroid surgery: experience of 2034 consecutive patients. J Otolaryngol Head Neck Surg 2014;43:16-23. [Crossref] [PubMed]
- De Falco M, Santangelo G, Del Giudice S, et al. Double probe intraoperative neuromonitoring with a standardized method in thyroid surgery. Int J Surg 2014;12:S140-4. [Crossref] [PubMed]
- Melin M, Schwarz K, Pearson MD, et al. Postoperative vocal cord dysfunction despite normal intraoperative neuromonitoring: an unexpected complication with the risk of bilateral palsy. World J Surg 2014;38:2597-602. [Crossref] [PubMed]
- Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg 2014;38:599-606. [Crossref] [PubMed]
- Schneider R, Sekulla C, Machens A, et al. Postoperative vocal fold palsy in patients undergoing thyroid surgery with continuous or intermittent nerve monitoring. Br J Surg 2015;102:1380-7. [Crossref] [PubMed]
- Cavicchi O, Burgio L, Cioccoloni E, et al. Intraoperative intermittent neuromonitoring of inferior laryngeal nerve and staged thyroidectomy: our experience. Endocrine 2018;62:560-5. [Crossref] [PubMed]
- Sedlmaier A, Steinmüller T, Hermanns M, et al. Continuous versus intermittent intraoperative neuromonitoring in complex benign thyroid surgery: A retrospective analysis and prospective follow-up. Clin Otolaryngol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-98. [Crossref] [PubMed]
- Schneider R, Sekulla C, Machens A, et al. Dynamics of loss and recovery of the nerve monitoring signal during thyroidectomy predict early postoperative vocal fold function. Head Neck 2016;38:E1144-51. [Crossref] [PubMed]
- Schneider R, Randolph GW, Barczynski M, et al. Continuous intraoperative neural monitoring of the recurrent nerves in thyroid surgery: a quantum leap in technology. Gland Surgery 2016;5:607-16. [Crossref] [PubMed]
- Schneider R, Machens A, Sekulla C, et al. Twenty-year experience of paediatric thyroid surgery using intraoperative nerve monitoring. Br J Surg 2018;105:996-1005. [Crossref] [PubMed]
- Wu CW, Wang MH, Chen CC, et al. Loss of signal in recurrent nerve neuromonitoring: causes and management. Gland Surg 2015;4:19-26. [PubMed]
- Schneider R, Randolph G, Dionigi G, et al. Prospective study of vocal fold function after loss of the neuromonitoring signal in thyroid surgery: The International Neural Monitoring Study Group’s POLT Study. Laryngoscope 2016;126:1260-6. [Crossref] [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents RLN paralysis by revealing initial EMG changes of impending neuropraxic injury: A prospective, multicenter study. Laryngoscope 2014;124:1498-505. [Crossref] [PubMed]
- Schneider R, Machens A, Randolph G, et al. Impact of continuous intraoperative vagus stimulation on intraoperative decision making in favor of or against bilateral surgery in benign goiter. Best Pract Res Clin Endocrinol Metab 2019;33:101285. [Crossref] [PubMed]
- Schneider R, Randolph G, Dionigi G, et al. Prediction of postoperative vocal fold function after intraoperative recovery of loss of signal. The International Neuromonitoring Study Group’s PREC Study. Laryngoscope 2019;129:525-31. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Loss of the nerve monitoring signal during bilateral thyroid surgery. Br J Surg 2012;99:1089-95. [Crossref] [PubMed]
- Schneider R, Lorenz K, Sekulla C, et al. Surgical strategy during intended total thyroidectomy after loss of EMG signal on the first side of resection. Chirurg 2015;86:154-63. [Crossref] [PubMed]
- Wu CW, Sun H, Zhang G, et al. Staged Thyroidectomy: A Single Institution Perspective. Laryngoscope Investig Otolaryngol 2018;3:326-32. [Crossref] [PubMed]
- Schneider R, Randolph GW, Dionigi G, et al. International neural monitoring study group guideline 2018 part I: Staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope 2018;128:S1-17. [Crossref] [PubMed]
- Sitges-Serra A, Gallego-Otaegui L, Fontané J, et al. Contralateral surgery in patients scheduled for total thyroidectomy with initial loss or absence of signal during neural monitoring. Br J Surg 2019;106:404-11. [Crossref] [PubMed]
- Bartsch DK, Dotzenrath C, Vorländer C, et al. Current Practice of Surgery for Benign Goitre-An Analysis of the Prospective DGAV StuDoQ|Thyroid Registry. J Clin Med 2019. [Crossref] [PubMed]
- Marti JL, Holm T, Randolph G. Universal Use of Intraoperative Nerve Monitoring by Recently Fellowship-Trained Thyroid Surgeons is Common, Associated with Higher Surgical Volume, and Impacts Intraoperative Decision-Making. World J Surg 2016;40:337-43. [Crossref] [PubMed]
- Feng AL, Puram SV, Singer MC, et al. Increased prevalence of neural monitoring during thyroidectomy: Global surgical survey. Laryngoscope 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Duclos A, Lifante JC, Ducarroz S, et al. Influence of intraoperative neuromonitoring on surgeons' technique during thyroidectomy. World J Surg 2011;35:773-8. [Crossref] [PubMed]
- Alesina PF, Hinrichs J, Meier B, et al. Intraoperative neuromonitoring for surgical training in thyroid surgery: its routine use allows a safe operation instead of lack of experienced mentoring. World J Surg 2014;38:592-8. [Crossref] [PubMed]
- Angelos P. Ethical and medicolegal issues in neuromonitoring during thyroid and parathyroid surgery: a review of the recent literature. Curr Opin Oncol 2012;24:16-21. [Crossref] [PubMed]
- Demontis R, Pittau MR, Maturo A, et al. Medico legal aspects on neuromonitoring in thyroid surgery: informed consent on malpractice claims. G Chir 2017;38:149-54. [Crossref] [PubMed]
- Verzeletti A, Vassalini M, Bin P, et al. Malpractice claims related to recurrent laryngeal nerve injury: Forensic remarks regarding 15 cases. Egyptian J Foren Scien 2016;6:501-4. [Crossref]
- Uldry E, Schäfer M, Saadi A, et al. Patients' preferences on information and involvement in decision making for gastrointestinal surgery. World J Surg 2013;37:2162-71. [Crossref] [PubMed]
- Dralle H, Lorenz K, Machens A. Verdicts on malpractice claims after thyroid surgery: emerging trends and future directions. Head Neck 2012;34:1591-6. [Crossref] [PubMed]
- Gartland RM, Bloom JP, Parangi S, et al. Unnerving Road: Malpractice Claims Involving the Surgical Management of Thyroid and Parathyroid Disease. World J Surg 2019;43:2850-5. [Crossref] [PubMed]
- Dionigi G, Bacuzzi A, Boni L, et al. Visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy: what about the costs? World J Surg 2012;36:748-54. [Crossref] [PubMed]
- Gremillion G, Fatakia A, Dornelles A, et al. Intraoperative recurrent laryngeal nerve monitoring in thyroid surgery: is it worth the cost? Ochsner J 2012;12:363-6. [PubMed]
- Rocke DJ, Goldstein DP, de Almeida JR. A Cost-Utility Analysis of Recurrent Laryngeal Nerve Monitoring in the Setting of Total Thyroidectomy. JAMA Otolaryngol Head Neck Surg 2016;142:1199-205. [Crossref] [PubMed]
- Wang T, Kim HY, Wu CW, et al. Analyzing cost-effectiveness of neural-monitoring in recurrent laryngeal nerve recovery course in thyroid surgery. Int J Surg 2017;48:180-8. [Crossref] [PubMed]
- Al-Qurayshi Z, Kandil E, Randolph GW. Cost-effectiveness of intraoperative nerve monitoring in avoidance of bilateral recurrent laryngeal nerve injury in patients undergoing total thyroidectomy. Br J Surg 2017;104:1523-31. [Crossref] [PubMed]
- Vasileiadis I, Karatzas T. Cost-effectiveness of recurrent laryngeal nerve monitoring in thyroid surgery. Gland Surg 2019;8:307-11. [Crossref] [PubMed]
- Sun H, Wu CW, Zhang D, et al. New Paradigms for Neural Monitoring in Thyroid Surgery. Surg Technol Int 2019;34:79-86. [PubMed]


