
Surgical approach to patients with primary aldosteronism
Introduction
Primary hyperaldosteronism (PA) is characterized by an inappropriate production of aldosterone from one or both of the adrenal glands. It is one of the most common causes of endocrine-related hypertension and patients will often present with severe, medically resistant hypertension (1,2). Fortunately, approximately 40% of patients with PA will have unilateral disease, such as an aldosterone producing adenoma or unilateral adrenal hyperplasia, and thus be amenable to surgical cure (3-5). If unilateral disease is present, the patient should be considered for operative intervention (6,7). In order to determine this, the patient must undergo tumor lateralization. Noninvasive cross-sectional imaging, such as a CT or MRI scan, can be used as an initial diagnostic test; however, these tests may not identify small, subcentimeter tumors. Since many aldosteronomas are less than 1.5 cm, CT could fail to localize a tumor in up to 10–20% of patients (4-6,8,9). Furthermore, in 2010, Mathur et al. performed AVS after a CT scan on 114 patients with PA and found that CT findings can be misleading in 50% of patients. In this study, of 57 patients who had a unilateral abnormality on CT, 6 had bilateral hyperplasia and 5 had a contralateral abnormality on AVS. Thus, 11 of 57 patients with a unilateral abnormality on CT would have undergone unnecessary surgery or had the normal adrenal resected. The remaining 57 patients had either a bilateral abnormality or no abnormality on CT; however, 46 (80%) of those patients had unilateral localization on AVS. Consequently, 46 of 57 patients with bilateral abnormalities or no findings on CT would have a delay in appropriate surgical intervention, prolonging the deleterious effects of resistant hypertension (10).
Approximately 2–10% of patients who have a biochemical diagnosis of hyperaldosteronism and an adrenal mass on CT who proceed with surgical resection will have persistent hyperaldosteronism postoperatively. In these patients, the adrenal mass represents a nonfunctional cortical adenoma and the etiology of the hyperaldosteronism is either a contralateral microadenoma or bilateral adrenal hyperplasia (5,11). The incidence of non-functional cortical adenomas increases with age, from <0.2% among patients below 40 years of age, to 3–5% among patients age 40–70 and up to 7% for patients above the age of 70 (11-13).Consequently, if the CT is non-lateralizing or if the patient is older and there is a question of whether they may have a non-producing cortical adenoma, a more invasive lateralization study should be considered (4,5,11).
Arteriovenous sampling (AVS)
AVS is the most common invasive localization study for PA. AVS is performed by cannulating each adrenal vein, which is confirmed by a 5-fold increase in cortisol levels in the adrenal vein compared to the peripheral circulation (IVC), followed by measuring the aldosterone and cortisol levels from each adrenal vein. Successful lateralization occurs when there is a minimum of a four-fold increase in aldosterone to cortisol ratio from one side compared to the other (5,11). An international, multi-institutional study demonstrated that patients with unilateral PA diagnosed by AVS have a higher likelihood of achieving complete biochemical success, defined as normal suppression according to the Primary Aldosteronism Surgical Outcome (PASO) criteria, compared to patients diagnosed by CT alone (14).
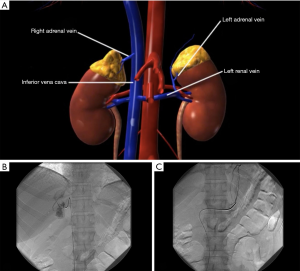
One disadvantage to AVS is the difficulty of performing the procedure. Failure rates of AVS can be as high as 80%, most commonly due to the difficulty of cannulating the right adrenal vein because of the sharp angle at which it enters the IVC (Figure 1) (4,5,11). Moreover, AVS is an invasive procedure that is not without risk, including, albeit rare, adrenal vein rupture (15). Furthermore, instrumentation of the adrenal vein can lead to a local inflammatory response and create adhesions that may make subsequent dissection more difficult. As a result, an institution may decide to proceed directly to unilateral adrenalectomy in a young patient (<35–40 years) with marked hypokalemia and a high aldosterone to renin ratio who has a large unilateral adrenal mass on CT (5,13,16).
Surgical approaches
Open vs. laparoscopic
Once the tumor has been localized, surgical options include open, laparoscopic, or robotic with an anterior, lateral, or posterior approach. Since laparoscopic adrenalectomy was first described almost 30 years ago, the literature has demonstrated the safety and effectiveness of laparoscopic adrenalectomy for adrenal disease (6,17-21). Specifically, laparoscopic adrenalectomy is associated with lower morbidity, decreased hospital stay, lower rates of ileus, and decreased postoperative pain compared to open adrenalectomy (20,22-25). The preferred approach for PA is the laparoscopic technique, which includes a transabdominal or retroperitoneoscopic approach.
Laparoscopic transabdominal adrenalectomy
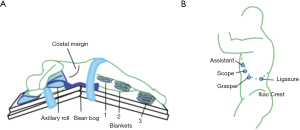
Transabdominal adrenalectomy can be performed via an anterior or lateral approach. With the advent of laparoscopy, the initial approach for adrenalectomy was an anterior transabdominal approach with the patient in a supine position. This provides a wide working area for the surgeon, familiar anatomic landmarks, and allows for bilateral adrenalectomy without repositioning (26). In 1992, Ganger described performing laparoscopic adrenalectomy with the patient in the lateral decubitus position. The patient is secured using a beanbag and the operating table is flexed at the waist in order to open the space between the lower rib and the iliac crest (Figure 2) (4,27). Raising a kidney rest can further expand this space. The lateral position has the advantage of gravitational autoretraction of intra-abdominal organs, which improves exposure and decreases risk of injury to surrounding structures (4,17,27).
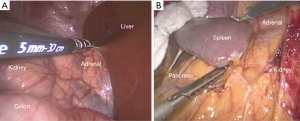
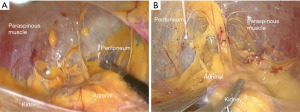
However, the patient would require repositioning if bilateral adrenalectomy were necessary (28). Furthermore, while there was a decreased risk of injury to surrounding structures, the lateral transabdominal technique requires mobilization of intra-abdominal organs including the colon, liver (on the right side), or spleen/pancreas (on the left side), which risks injury and can increase operative time (Figure 3) (29).
Retroperitoneoscopic adrenalectomy
In 1995, Mercan described the endoscopic posterior retroperitoneal approach, which provides for a more direct access to the adrenal gland (9,30). The patient is in a modified prone position with two non-compressible bolsters under the chest and hips, allowing the ventral wall of the abdomen to hang freely and thus avoiding compression of the retroperitoneal space (19,27). The bed is flexed to accentuate the space between the costal margin and the iliac crest and the legs are lowered to horizontally position the back parallel to the floor (Figure 4A) (4,27).
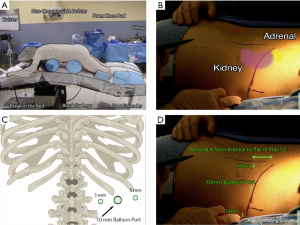
This approach can be performed using 3 ports, which is one of the most critical steps to prepare for a successful operation (19,31). The first port should be placed 2 cm below the tip of the 12th rib (32). Care must be taken not to go too close to the 12th rib or the patient may experience postoperative neuralgia. Furthermore, the port should enter the retroperitoneum at a 45-degree angle to the 12th rib to optimize the approach to the adrenal gland with rigid laparoscopic instruments (Figure 4B,C,D) (33).
By avoiding the intra-abdominal cavity, intra-abdominal adhesions from prior operations can be avoided and mobilization of intra-abdominal organs including the liver, colon, spleen and pancreas, to provide exposure is unnecessary (Figure 5) (9,26,28,29,31-34). With this approach, the patient can undergo bilateral adrenalectomy without the need for repositioning (9,28). Furthermore, compared to the lateral transabdominal approach, the retroperitoneoscopic approach is associated with less postoperative pain, lower analgesic requirements, and a shorter hospital stay (26,30).
However, there are several limitations to the retroperitoneoscopic approach. Although the access is more direct, the retroperitoneum is a smaller working space and the lack of familiar anatomic landmarks can be disorienting, especially for less experienced surgeons (9,28). The literature reports a learning curve of 20–25 cases required in order to complete the operation under 90 minutes (31,35). Due to the smaller working space, some prefer lateral transabdominal approach for tumors >6 cm; however, some series reported resecting tumors up to 8 cm in size with the retroperitoneoscopic approach (9,28,30,32-34). Patients with a higher BMI tend to have thick perinephric fat, which can make mobilization of the kidney more difficult (23,31,32). Although some report success in patients with higher BMI, most surgeons will prefer lateral transabdominal for a BMI >40 (34,36,37). Furthermore, studies have reported difficulty with the retroperitoneoscopic approach in patients with a large distance between the skin and Gerota’s fascia (>4 cm), which limits the ability of the instruments to dissect the adrenal gland (26,31).
Ultimately, both the lateral and posterior approaches are safe and effective. Furthermore, the majority of studies comparing the two approaches found no difference in operative time or morbidity. Thus, while there are certain factors that may make one laparoscopic approach more favorable to another, the choice of lateral transabdominal versus retroperitoneoscopic is often dependent on surgeon preference (28).
Postoperative considerations
In 2017, the Primary Aldosteronism Surgical Outcome (PASO) study published consensus guidelines for postoperative outcomes and management of patients with unilateral PA. These set of criteria consisted of clinical outcomes (blood pressure readings, use of antihypertensive medications) and biochemical outcomes (plasma concentrations of aldosterone, potassium, and renin). Patients were categorized as reaching complete, partial or absent biochemical and clinical success. The majority of patients from this study (94%) achieved complete biochemical success; however, less than half (37%) of patients achieved complete clinical success (defined as normotensive off of all antihypertensive medications) (38).
To assess the importance of a partial clinical success after unilateral adrenalectomy, another international multi-institutional study was performed. In the International CONNsortium study, researchers examined the outcomes for patients who remained hypertensive requiring antihypertensive medication postoperatively. Results demonstrated biochemical and clinical cure in 27% of patients, clear improvement in 31% and no clear improvement in 41.8%. However, of the patients with no clear success, the mean systolic and diastolic blood pressure decreased by 9 and 3 mmHg, respectively. Consequently, even those who are unable to wean off of their antihypertensives, there is still a potential benefit with surgery (1). In general, antihypertensive medications should be decreased in dose or stopped entirely postoperatively with close follow-up after discharge (4).
Due to the high rate of biochemical cure postoperatively, all potassium supplements and potassium-sparing diuretics should be stopped immediately to prevent hyperkalemia (4,8,27). Electrolytes should be checked on postop day 1 and then weekly for the subsequent 4 weeks (4,27).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vorselaars WM, Nell S, Postma EL, et al. Clinical Outcomes After Unilateral Adrenalectomy for Primary Aldosteronism. JAMA Surg 2019;154:e185842. [Crossref] [PubMed]
- Iacobone M, Citton M, Viel G, et al. Approach to the surgical management of primary aldosteronism. Gland Surg 2015;4:69-81. [PubMed]
- Vilela LAP, Almeida MQ. Diagnosis and management of primary aldosteronism. Arch Endocrinol Metab 2017;61:305-12. [Crossref] [PubMed]
- Lal G, Duh QY. Laparoscopic adrenalectomy--indications and technique. Surg Oncol 2003;12:105-23. [Crossref] [PubMed]
- Yeh M, Duh QY. The Adrenal Glands. In: Townsend CM Jr, Beauchamp RD, Evers BM, et al. Sabiston’s Textbook of Surgery. 18th edition. Berlin: Elsevier, 2007.
- Grant CS, Carpenter P, van Heerden JA, et al. Primary Aldosteronism. Arch Surg 1984;119:585-90. [Crossref] [PubMed]
- Goh BK, Tan YH, Yip SK, et al. Outcome of patients undergoing laparoscopic adrenalectomy for primary hyperaldosteronism. JSLS 2004;8:320-5. [PubMed]
- Rossi H, Kim A, Prinz RA. Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg 2002;68:253-6; discussion 256-7. [PubMed]
- Siperstein AE, Berber E, Engle KL, et al. Laparoscopic posterior adrenalectomy: technical considerations. Arch Surg 2000;135:967-71. [Crossref] [PubMed]
- Mathur A, Kemp CD, Dutta U, et al. Consequences of adrenal venous sampling in primary hyperaldosteronism and predictors of unilateral adrenal disease. J Am Coll Surg 2010;211:384-90. [Crossref] [PubMed]
- Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004;136:1227-35. [Crossref] [PubMed]
- Kloos RT, Gross MD, Francis IR, et al. Incidentally discovered adrenal masses. Endocr Rev 1995;16:460-84. [PubMed]
- Young WF Jr. Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am 2000;29:159-85. x. [Crossref] [PubMed]
- Williams TA, Burrello J, Sechi LA, et al. Computed Tomography and Adrenal Venous Sampling in the Diagnosis of Unilateral Primary Aldosteronism. Hypertension 2018;72:641-9. [Crossref] [PubMed]
- Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab 2012;97:1606-14. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolte E, et al. Laparoscopic Adrenalectomy. Surg Endosc 1994;8:135-8. [Crossref] [PubMed]
- Tullavardhana T. Laparoscopic Adrenalectomy: Surgical Technique. WJOLS 2010;3:91-7. [Crossref]
- Walz MK, Gwosdz R, Levin SL, et al. Retroperitoneoscopic adrenalectomy in Conn's syndrome caused by adrenal adenomas or nodular hyperplasia. World J Surg 2008;32:847-53. [Crossref] [PubMed]
- Heslin MJ, Winzeler AH, Weingarten JO, et al. Laparoscopic adrenalectomy and splenectomy are safe and reduce hospital stay and charges. Am Surg 2003;69:377-81. [PubMed]
- Jacobs JK, Goldstein RE, Geer RJ. Laparoscopic adrenalectomy. A new standard of care. Ann Surg 1997;225:495-501; discussion 501-2. [Crossref] [PubMed]
- Elfenbein DM, Scarborough JE, Speicher PJ, et al. Comparison of laparoscopic versus open adrenalectomy: results from American College of Surgeons-National Surgery Quality Improvement Project. J Surg Res 2013;184:216-20. [Crossref] [PubMed]
- Hazzan D, Shiloni E, Golijanin D, et al. Laparoscopic vs open adrenalectomy for benign adrenal neoplasm. Surg Endosc 2001;15:1356-8. [Crossref] [PubMed]
- Wang HS, Li CC, Chou YH, et al. Comparison of laparoscopic adrenalectomy with open surgery for adrenal tumors. Kaohsiung J Med Sci 2009;25:438-44. [Crossref] [PubMed]
- Duncan JL 3rd, Fuhrman GM, Bolton JS, et al. Laparoscopic adrenalectomy is superior to an open approach to treat primary hyperaldosteronism. Am Surg 2000;66:932-5; discussion 935-6. [PubMed]
- Lee CR, Walz MK, Park S, et al. A comparative study of the transperitoneal and posterior retroperitoneal approaches for laparoscopic adrenalectomy for adrenal tumors. Ann Surg Oncol 2012;19:2629-34. [Crossref] [PubMed]
- Madani A, Lee JA. Surgical Approaches to the Adrenal Gland. Surg Clin North Am 2019;99:773-91. [Crossref] [PubMed]
- Duh QY, Siperstein AE, Clark OH, et al. Laparoscopic adrenalectomy. Comparison of the lateral and posterior approaches. Arch Surg 1996;131:870-5; discussion 875-6. [Crossref] [PubMed]
- Berber E, Duh QY, Clark OH, et al. A critical analysis of intraoperative time utilization in laparoscopic adrenalectomy. Surg Endosc 2002;16:258-62. [Crossref] [PubMed]
- Mercan S, Seven R, Ozarmagan S, et al. Endoscopic retroperitoneal adrenalectomy. Surgery 1995;118:1071-5; discussion 1075-6. [Crossref] [PubMed]
- Berber E, Tellioglu G, Harvey A, et al. Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery 2009;146:621-5; discussion 625-6. [Crossref] [PubMed]
- Bonjer HJ, Bruining HA. Endoscopic retroperitoneal-flank approach. Oper Tech Gen Surg 2002;4:322-30. [Crossref]
- Agcaoglu O, Sahin DA, Siperstein A, et al. Selection algorithm for posterior versus lateral approach in laparoscopic adrenalectomy. Surgery 2012;151:731-5. [Crossref] [PubMed]
- Vrielink OM, Wevers KP, Kist JW, et al. Laparoscopic anterior versus endoscopic posterior approach for adrenalectomy: a shift to a new golden standard? Langenbecks Arch Surg 2017;402:767-73. [Crossref] [PubMed]
- Barczyński M, Konturek A, Gołkowski F, et al. Posterior retroperitoneoscopic adrenalectomy: a comparison between the initial experience in the invention phase and introductory phase of the new surgical technique. World J Surg 2007;31:65-71. [Crossref] [PubMed]
- Walz MK, Alesina PF, Wenger FA, et al. Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery 2006;140:943-8; discussion 948-50. [Crossref] [PubMed]
- Dickson PV, Jimenez C, Chisholm GB, et al. Posterior retroperitoneoscopic adrenalectomy: a contemporary American experience. J Am Coll Surg 2011;212:659-65; discussion 665-7. [Crossref] [PubMed]
- Williams TA, Lenders JW, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol 2017;5:689-99. [Crossref] [PubMed]


