Laparoscopic adrenalectomy
Introduction
Gagner et al. first described trans-abdominal laparoscopic adrenalectomy with the flank approach in the lateral decubitus position in 1992 (1). Subsequently, the technique has been further standardized (2,3) and quickly became the gold standard treatment for most surgical adrenal disorders (4,5).
The successful application of minimally invasive surgery to adrenals is mainly due to some key factors: the endoscopic approach allows an optimal exposure of the adrenal area; the magnification provided by the endoscope is particularly helpful during the dissection of an anatomically complex and dangerous region as retroperitoneum is; from an anatomically point of view the adrenal vascular supply is well defined; the adrenalectomy is an ablative procedure, thus particularly suitable for an endoscopic approach (6).
Several retrospective and comparative studies addressed the advantages of minimally invasive adrenalectomy specifically consistent in less postoperative pain, improved patients’ satisfaction, shorter hospital stay and recovery time when compared to open adrenalectomy (5,7-12).
These results have been more recently, validated by several USA national surveys that confirmed that laparoscopic adrenalectomy has significantly lower perioperative morbidity and shorter length of hospital stay than open adrenalectomy (13-15).
The laparoscopic transabdominal lateral adrenalectomy (TLA) is currently the most widely used approach, since it allows an optimal comprehensive view of the adrenal lodge and surrounding structures, and provides adequate working space. An additional advantage of the transabdominal approach is the possibility to explore the abdominal cavity allowing the treatment of eventually associated abdominal pathologies during the same procedure. Moreover, this approach allows a quick conversion to hand-assisted or open surgery in the case of difficult dissection or intraoperative haemorrhage.
However, previous abdominal surgery particularly when performed on the retroperitoneal area (kidney, pancreas, or spleen) can produce significant adhesions in the adrenal region and may render the trans-abdominal approach challenging particularly for surgeons with limited laparoscopic experience. Despite this, several series reported that up to 55% of patients had previous abdominal surgery but conversions to open surgery were very rarely attributed to adhesions (5,16,17).
The aim of this article is to review briefly the experience gained with TLA, and to evaluate its effectiveness for the surgical management of adrenal disease.
Operative techniques
One of the main advantages of the trans-abdominal lateral approach is to allow the gravity-facilitated exposure of the adrenals (2,3,6). Indeed, after the mobilization of the structures overlying the adrenals, the liver on the right, and the spleen and tail of the pancreas on the left, there is no need to manipulate further these structures during the following steps of the procedure.
From a technical point of view, essential requirements for a successful laparoscopic adrenalectomy are an appropriate knowledge of retroperitoneal anatomy, a gentle tissue manipulation and a precise haemostasis technique in order to adequately identify the structures of interest and avoid the troublesome oozing that could complicate the surgical procedure (2,3,6).
Patient and trocars position
The TLA requires general anaesthesia, with muscle relaxation and controlled ventilation. The operating table should be capable of flexion with a kidney rest that can be elevated. The patient should be placed initially in a supine position for induction anaesthesia. An orogastric tube for gastric decompression (mainly helpful in left-sided adrenalectomy) and a Foley catheter are usually placed and generally removed at the end of the procedure. The current guidelines for antibiotic prophylaxis (18) and for prevention of venous thromboembolism (19) are applicable to most of adrenal pathologies, whereas some diseases (e.g., Cushing) are associated with a higher operative and perioperative risk (20).
Atraumatic graspers, scissor, hook, and clip applier are common to many laparoscopic procedures. More specific for adrenalectomy are small swabs, allowing atraumatic retraction of the gland. A right-angled grasper or vascular clamp should be ready on the operative table. An atraumatic grasper is useful for the mobilization of the adrenal gland in order to avoid bleeding during the manipulation of periadrenal fat. A needle holder must be available to perform laparoscopic suturing if required to repair vessel injury. Safe dissection requires a high-quality CCD camera. The operation is performed using a 0-30 degree 5–10-mm laparoscope.
The patient is turned in a full lateral left decubitus position for the right and in a full lateral right decubitus position for the left adrenalectomy respectively, with the 10th rib directly over the breakpoint in the table. A cushion is placed under the opposite flank with respect to the side of adrenalectomy. The table is flexed in order to maximize the exposure of the space between the costal margin and the iliac crest, avoiding an excessive tension of the abdominal wall, which may decrease its distensibility during CO2 insufflations. The right/left arm is elevated and secured on an elevated arm board. The patient’s legs are flexed to avoid stretching of the crural nerve. The area from the umbilicus to the spine and from the nipple down to the superior anterior iliac crest should be exposed. Adequate patient positioning is essential for technical success in laparoscopic adrenalectomy (2). The surgeons stand on the abdominal side of the patient, facing the monitor at the head of the patient.
Initial peritoneal access is achieved 2 cm inferior to the right/left costal margin in the midclavicular line, with either the blind (Verres Needle) access, with the open (Hasson) access or with the optical access trocar (1-3,5,6,21). The Verres technique implies CO2 insufflation starting in the right/left subcostal area with a Verres needle up to 15 mmHg. The Verres needle is placed under the right/left costal margin at the anterior axillary line and lateral to the rectus muscle. It is mandatory to perform a saline test in order to exclude organs injuries. Otherwise, pneumoperitoneum is induced by an open approach at the site of the first trocar. Optical access trocars allow inserting the endoscope directly inside the clear tip trocar, enabling the surgeon to visualize all the abdominal layers during port placement. A pressure of 12–14 mmHg is generally used for CO2 insufflation.
Right adrenalectomy
A 10–12 mm trocar for the endoscope is placed in the subcostal area in the anterior axillary line. A diagnostic laparoscopy is then performed. The ascending colon, the liver, the right kidney, the diaphragm, and the duodenum are inspected. If there are signs suggestive of adrenal malignancies (e.g., local invasion, though this is rarely apparent at this stage in the procedure) conversion is mandatory.
Under direct vision, the second 10–12 mm trocar is placed in the subcostal area medially to the first one. This receives graspers for exposure of the operative field, hook, scissors, retractors, instruments with peanut swabs and energy devices to achieve adequate haemostasis. The third trocar (5 mm) is inserted between the anterior axillary line and the epigastrium, receiving a smooth retractor in order to retract the liver during the whole procedure. The fourth trocar (5 mm) is inserted at the subcostal angle (Figure 1).
Left adrenalectomy
Left TLA may be performed with three trocars in most of cases, although a forth additional port can be optional (Figure 2). A 10 mm trocar is positioned in the subcostal space at the anterior axillary line for the endoscope. Diagnostic laparoscopy is then performed. The ligament of the colonic splenic flexure and the descending colon are inspected. The spleen, the lateral segments of the left liver, the diaphragm, and the greater curvature of the stomach are inspected. If there are signs suggestive of adrenal malignancies (e.g., local invasion) conversion is mandatory. If the inspection is satisfactory, two other 5–10 mm trocars are placed under direct vision about 7 cm on each side of the first trocar below the costal margin. As in the right side, they will take graspers for exposure of the operative field, hook, scissors, retractors, instruments with peanut swabs and energy devices to achieve adequate haemostasis. The forth trocar, when necessary, is positioned below the first one, at distance of 4 to 5 cm.
Right TLA: surgical steps (Figure 3)

Exposure
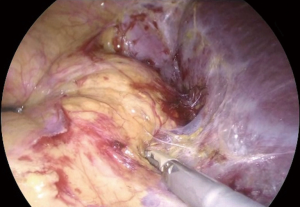
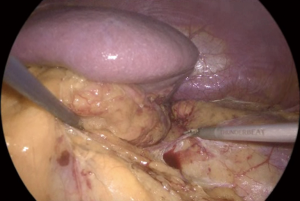
The key factor for an adequate exposure is an effective dissection of the liver right triangular ligament and of the hepatoparietal ligament wide enough in order to achieve a complete mobilization of the liver, that can be retracted upwards and medially (Figure 4). After the effective liver mobilization, the adrenal gland and the inferior vena cava are adequately exposed (Figure 5).
Dissection of the main vein
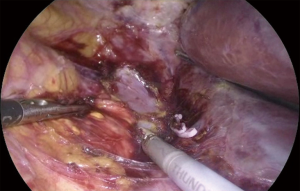
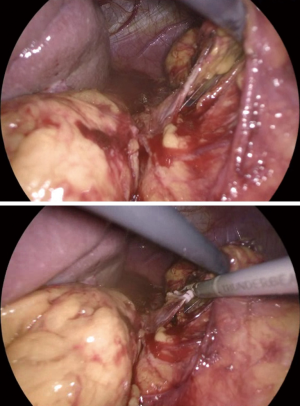
Once, the medial edge of the adrenal gland is identified, the plane between the vena cava and the gland is opened (Figure 6), allowing the lateral retraction of the adrenal and thus exposing the area where the main adrenal vein runs. The main landmark for the identification of the right adrenal vein is the inferior vena cava. The dissection of the lateral edge of the vena cava should carry out starting from the right renal vein and heading superiorly. Once the main adrenal vein is identified and dissected by the means of a right angled (Figure 6), it is doubly clipped and divided, completing the most difficult step of the dissection (Figure 7). The dissection of the adrenal vein as first step of the adrenalectomy, can be more demanding in case of large size adrenal lesion. Indeed, in this case can be suitable starting the dissection from the lateral and superior aspect of the lesion and then moving inferiorly along the vena cava. In about 20% of cases, an accessory adrenal vein is encountered 2–3 cm above the main adrenal vein and when present should be dissected, clipped, and divided.
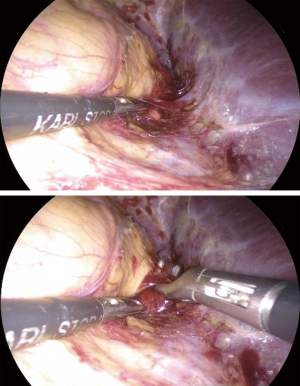
End of the dissection/extraction
The adrenalectomy then proceeds with the dissection of the inferior aspect of the adrenal en bloc with the periadrenal fat. Than the adrenal is lifted up and the dissection is continued at the posterior and lateral aspect of the gland and finally superiorly. The last step of dissection is the identification and the division of the three main adrenal arteries and accessory veins. The adrenal within the retrieval bag is removed through a 10–12 mm trocar. Trocar sites can be slightly enlarged if needed. The placement of a drain in the adrenal lodge is optional but generally advisable. Careful port site closure is recommended in order to prevent incisional hernias.
Pitfalls
Besides the general pitfalls related to the laparoscopic approach (bowel and vascular injuries, gas embolism, operative difficulties linked to adhesions, obesity, etc.), in the right adrenalectomy there are some specific side-related problems as: liver injury; duodenum injury; vena cava injury; division of a polar renal artery; rupture of the adrenal capsule; injury of the diaphragm.
Left TLA: surgical steps (Figure 8)

Several factors as the lack of major anatomic landmark (e.g., the inferior vena cava in the right side), the relative small size of the left adrenal gland, the main vein within the retroperitoneal fat and the close proximity of the pancreas tail, may render the left adrenalectomy a challenging procedure.
The prerequisite in order to achieve an adequate exposure of the left adrenal gland is a complete mobilization of the splenopancreatic bloc. Indeed, an effective dissection of the spleen along with the tail of the pancreas allow to take advantage of the gravity-facilitated exposure of the left adrenal, since the spleen will fall away from the operative field.
Exposure
The first step of adrenalectomy is the dissection of the left colonic flexure (Figure 9).
Afterwards, the next step of the procedures is the mobilization of the spleen, accomplished by dissecting the splenoparietal ligament (Figure 10). The lateral decubitus position allows for an easy exposure of the splenoparietal ligament. The dissection of the splenoparietal ligament is starting at posterior and inferior edge of the spleen, taking care to left a margin of about 2 cm of peritoneum for an effective retraction of the spleen allowing the exposition of its posterior aspect. The splenoparietal ligament dissection is continued until the diaphragm, far enough to visualize the fundus of the stomach (Figure 10) and the left crus of the diaphragm.
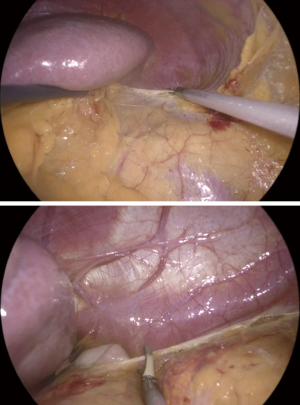
The full dissection of the splenoparietal ligament allows a complete mobilization of the spleen.
Then, the dissection proceeds with the dissection of the splenorenal ligament, starting from the posterior aspect of the spleen and continuing with the tail of the pancreas. The medial and anterior retraction of the splenorenal ligament allows its dissection in a superficial plane, avoiding the deep dissection in the perirenal fat. At this point, the splenopancreatic bloc is displaced medially, out of the operative field, with gravity playing a major role (Figure 11), and the kidney upper pole and the adrenal area are exposed.
Dissection of the main vein
The dissection of the left adrenal should start on the medial aspect of the gland proceedings from upper to lower adrenal pole, keeping close to the posterior muscular plane. This manoeuvre allows the lateral rotation of the adrenal and exposes the space where the left adrenal vein runs. The dissection of the lateral aspect of the gland should be avoided, since the adrenal would fall medially preventing the access to the medial and inferior edge of the gland. During the dissection of the medial aspect of the adrenal gland the diaphragmatic vein is often encountered: it represents an important landmark for the identification of the main left adrenal vein. Once the main adrenal vein is identified, it is isolated, often using a right-angled dissector, and doubly clipped and divided (Figure 12).
End of the dissection/extraction
After the dissection of the main adrenal vein, the adrenal en bloc with the periadrenal fat is lifted up, and the dissection continues at the posterior and lateral aspect of the gland. The adrenal upper pole is dissected lastly, allowing the ‘hanging technique’. Dissection can be performed using a hook, coagulating scissors or energy devices. The adrenal within the retrieval bag is removed through a 10–12 mm trocar (the trocar site can be enlarged if needed). The placement of a drain in the adrenal lodge is optional but generally advisable. Careful port site closure is recommended in order to prevent incisional hernias.
Pitfalls
Specific side-related problems that can be observed for a left adrenalectomy are splenic injury and pancreatic injury. In the left sided lesion, moreover, confusion can occur between the main adrenal vein and the renal vein especially in the case of large adrenal tumours that can displaced horizontally the generally oblique left adrenal vein. As in the right, also in the left adrenalectomy inadvertent division of an unrecognized polar renal artery, rupture of the capsule of the gland and diaphragmatic injury can occur.
Indications to TLA
Endoscopic adrenalectomy is the gold standard treatment for small to medium-sized (≤6 cm) benign adrenal tumours, both functioning and non-functioning (5,6,24).
However, the increasing experience with the endoscopic adrenalectomy produced the broadening of the indications to this approach, proposing it also for large and potentially malignant adrenal tumours (25,26).
Despite tumour size is usually considered a parameter predicting the malignancy of the adrenal lesion, it remains relatively insensitive and nonspecific (25). Indeed, the role of tumour size as a limiting factor for the choice of the surgical approach for adrenalectomy, seems unimportant for some surgeons (25-28). Conversely, other surgeons consider the tumours size as a key factor for the laparoscopic approach to adrenalectomy, assessing the adrenal lesion size threshold for endoscopic adrenalectomy between 6 and 10 cm (27,29-32). From a theoretical point of view, about 75% of adrenal tumours >6 cm will be benign at the final pathological report (28). Thus, if a tumour size >6 cm is recognized as a contraindication to laparoscopic adrenalectomy, the advantages of minimally invasive approach will be denied to patients having a most likely benign disease (27,33).
Moreover, the early experience on laparoscopic adrenalectomy reported that in experienced hands the endoscopic removal of large adrenal lesions (up to 10 cm in maximum diameter), in absence of suspicious radiological findings, was feasible and safe (5,25,34).
However, in the case of invasive adrenocortical carcinoma (ACC), open adrenalectomy remains the procedure of choice (27,35-42).
The large diffusion of minimally invasive adrenalectomy led to an increased referral to surgery in the case of adrenal incidentaloma (43), with a consequent increased risk of unexpected pathological diagnosis of ACC after endoscopic adrenalectomy (44). Indeed, the reported frequency of ACC in patients operated for adrenal incidentaloma reaches 10% in some series (45).
However, in absence of radiological suspicious findings (invasion of surrounding structures, lymph node or distant metastases, intravenous thrombus), it may difficult to predict malignancy pre-and even intra-operatively (45).
A complete surgical resection is the mainstay treatment of localized ACC [European Network for Study of Adrenal Tumors (ENSAT) stage I–III] (46), since a R0 resection is the only means to achieve long-term disease control in ACC patients (40,47). Some reports reported an increased risk of R1-R2 resection or tumour spill (44), peritoneal carcinomatosis (48,49) and earlier recurrence (44) in patients undergoing endoscopic adrenalectomy for localized ACC. Therefore, based on these findings, an international consensus conference on ACC strongly discouraged endoscopic adrenalectomy for the treatment of known or suspicious ACC (50).
On the contrary, recently published comparative studies based on single center (51) or multi-institutional series (52) demonstrated that the oncologic outcomes of localized ACC following endoscopic adrenalectomy and open adrenalectomy could be similar. Therefore, the role of endoscopic adrenalectomy in the treatment of localized ACC is one of the most controversial and debated topics in adrenal surgery.
Due to the low incidence of ACC, there are no randomized trials comparing endoscopic and open adrenalectomy (42). Indeed, the discussion on this subject should be on the basis of the retrospective study of single center series and multi-institutional surveys.
During the last years, several papers further supported the debate. Several series from the USA persist to discourage endoscopic adrenalectomy in patients with known or suspected ACC (53-56), while some reports from Europe showed that endoscopic adrenalectomy does not jeopardize the oncologic outcome of selected cases of ACC (57-59).
Therefore, nowadays, there are not definitive conclusion regarding the oncologic outcome of endoscopic adrenalectomy vs. open adrenalectomy in patients with ACC.
However, it could be argued that in referral centers the oncologic outcome of ACC treated with endoscopic approach is not inferior to that achieved whit open adrenalectomy, when strict selection criteria and the principles of oncologic surgery are observed. On the other hand, if performed by non-experienced surgeons, endoscopic adrenalectomy for ACC can involve a higher risk of R1/R2 resection and tumour bed and/or intraperitoneal recurrence, mostly if strict selection criteria and the rule of conversion to open approach in case of challenging dissection are not followed.
However, if an endoscopic approach is considered for an adrenal tumour at increased risk of malignancy (a mass with radiological intratumoral signs of suspicion and without clear locoregional involvement), the transabdominal lateral adrenalectomy might be preferred approach because it might allow intraoperative evaluation of the presence of distant metastasis and larger en bloc resection of the tumour (42).
Operative and post-operative outcomes of TLA
The majority of studies have demonstrated that laparoscopic adrenalectomy by transabdominal lateral approach is a safe technique with low perioperative complications and rare postoperative mortality (16,60-73).
The average complication rate reported for TLA is difficult to evaluate because of the lack of standardized definition through the different studies. However, the average rate of complications seems to be less than 9%, with a range between 2.9% and 15.5% (5,16,61,63-65,69-73).
Several risk factors for complications and conversion, as surgeon and hospital volume (60-66), tumour- and patients-related characteristics (16,67-73), have been evaluated in single-center (16,64,70,71,73) and national studies (60-63,65-68,72).
The impact of surgeon and hospital volumes on postoperative outcomes for adrenalectomy appears relevant in different experiences (60-66). Park et al. (61) in a population-based retrospective analysis including 3,144 adrenalectomies, observed a significantly higher rate of complications (18.3% vs. 11.3%) and a significantly longer hospital stay (5.5 vs. 3.9 days) in procedures performed by low-volume surgeons.
In a national study by Palazzo et al. (65) the authors found a mean hospital stay and a rate of 30-day readmissions significantly higher in the low- versus high-volume adrenal surgeons. Bergamini et al. (63) found that age, patients BMI, tumour size and diagnosis of phaeochromocytoma are risk factors for complications but observed a significantly lower rate of these complications in referral with the respect of non-referral centers.
In contrast, Gallagher et al. (66) did not found any association between surgeon volume and complication rates or length of hospital stay. However, the definition of high- versus low-volume surgeon is highly variable across the different study, probably due to the lack of a method to set a volume threshold. A recent USA national-level analysis conducted on a large series of patients who underwent adrenalectomy, showed that higher surgeon volume was associated with better patients’ outcomes and lower costs, suggesting an annual threshold of adrenalectomy ≥6 (60).
Among the patient’s characteristic affecting the TLA operative outcome, the most relevant risk factors for complications and conversion were obesity (16,73,74), history of previous abdominal surgery (16,71,75), the tumour side (69), patients’ comorbidities (73) and the diagnosis of pheochromocytoma (73).
Obesity with a body mass index ≥30 has been previously reported as risk factor of complication in laparoscopic adrenalectomy (74). However, more recently, it has been demonstrated that obesity is not associated with complications or prolonged length of hospital stay, but it significantly affects the operative time (16,73).
The history of abdominal surgery, especially previous upper mesocolic or retroperitoneal surgery, has been reported to increase the risk of intra- and post-operative complications as well as the risk of conversion (71,75). However, recently published study, did not find higher conversion and complications rate for TLA in patients who underwent previous abdominal surgery (16).
In a recent study, conversion to open surgery and left-sided adrenalectomy were founded to be independent risk factors for complications (69). The authors ascribed the finding of higher overall complications in left-sided tumours to the partial mobilization of the left pancreas and spleen required in left TLA (69).
The diagnosis of pheochromocytoma (69) and the patients’ comorbidities (73) have been also reported as risk factors for post-operative complications.
Postoperative complications were reported to be higher in patients with tumour size ≥45 mm (71) and ≥6 cm respectively (73). However, no differences in terms of conversion and complication rate were found in a comparative analysis of TLA performed with different cut-off of adrenal lesion size (<6 vs. 6–8 vs. >8 cm) (76).
Overall, conversion of TLA to an open procedure occurs in approximately 2% of cases, with a wide range between 0% and 13% (5,16,61,63-65,69-73). The most frequent reported causes of conversion are vascular or organ injury and technical difficulties (5,16,61,63-65,69-73).
The mortality rate of TLA, even if a standard definition is lacking across the different study, is low and appeared to be between 0% to 0.8% (5,16,61,63-65,69-73). The most frequent reported causes of mortality included massive bleeding, pancreatitis, pulmonary embolism, sepsis.
Conclusions
Minimally invasive adrenalectomy has become the standard approach for adrenalectomy in the proper clinical settings. The TLA has been shown to be safe and effective for most adrenal pathologies. Overall, the excellent results reported in the literature reflect the experience accumulated with TLA that remains an approach as relevant today as it was 25 years ago.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Gagner M, Lacroix A, Boltè E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolte E, et al. Laparoscopic adrenalectomy. The importance of a flank approach in the lateral decubitus position. Surg Endosc 1994;8:135-8. [Crossref] [PubMed]
- Marescaux J, Mutter D, Wheeler MH. Laparoscopic right and left adrenalectomies. Surgical procedures. Surg Endosc 1996;10:912-5. [Crossref] [PubMed]
- Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg 1999;23:389-96. [Crossref] [PubMed]
- Assalia A, Gagner M. Laparoscopic adrenalectomy. Br J Surg 2004;91:1259-74. [Crossref] [PubMed]
- Henry JF. Minimally invasive adrenal surgery. Best Pract Res Clin Endocrinol Metab 2001;15:149-60. [Crossref] [PubMed]
- Prinz RA. A comparison of laparoscopic and open adrenalectomies. Arch Surg 1995;130:489-92. [Crossref] [PubMed]
- Brunt LM, Doherty GM, Norton JA, et al. Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms. J Am Coll Surg 1996;183:1-10. [PubMed]
- Thompson GB, Grant CS, van Heerden JA, et al. Laparoscopic versus open posterior adrenalectomy: a case-control study of 100 patients. Surgery 1997;122:1132-6. [Crossref] [PubMed]
- Dudley NE, Harrison BJ. Comparison of open posterior versus transperitoneal laparoscopic adrenalectomy. Br J Surg 1999;86:656-60. [Crossref] [PubMed]
- Imai T, Kikumori T, Ohiwa M, et al. A case-controlled study of laparoscopic compared with open lateral adrenalectomy. Am J Surg 1999;178:50-3. [Crossref] [PubMed]
- Hallfeldt KK, Mussack T, Trupka A, et al. Laparoscopic lateral adrenalectomy versus open posterior adrenalectomy for the treatment of begin adrenal tumors. Surg Endosc 2003;17:264-7. [Crossref] [PubMed]
- Lee J, El-Tamer M, Schifftner T, et al. Open and laparoscopic adrenalectomy: analysis of the National Surgical Quality Improvement Program. J Am Coll Surg 2008;206:953-9. [Crossref] [PubMed]
- Elfenbein DM, Scarborough JE, Speicher PJ, et al. Comparison of laparoscopic versus open adrenalectomy: results from American College of Surgeons-National Surgery Quality Improvement Project. J Surg Res 2013;184:216-20. [Crossref] [PubMed]
- Eichhorn-Wharry LI, Talpos GB, Rubinfeld I. Laparoscopic versus open adrenalectomy: another look at outcome using the Clavien classification system. Surgery 2012;152:1090-5. [Crossref] [PubMed]
- Economopoulos KP, Phitayakorn R, Lubitz CC, et al. Should specific patient clinical characteristics discourage adrenal surgeons from performing laparoscopic transperitoneal adrenalectomy? Surgery 2016;159:240-8. [Crossref] [PubMed]
- Morris L, Ituarte P, Zarnegar R, et al. Laparoscopic adrenalectomy after prior abdominal surgery. World J Surg 2008;32:897-903. [Crossref] [PubMed]
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152:784-91. [Crossref] [PubMed]
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e691S-736S.
- Babic B, De Roulet A, Volpe A, et al. Is VTE Prophylaxis Necessary on Discharge for Patients Undergoing Adrenalectomy for Cushing Syndrome? J Endocr Soc 2018;3:304-13. [Crossref] [PubMed]
- Gaunay GS, Elsamra SE, Richstone L. Trocars: Site Selection, Instrumentation, and Overcoming Complications. J Endourol 2016;30:833-43. [Crossref] [PubMed]
- Raffaelli M, De Crea C, Bellantone R. Real time laparoscopic lateral transabdominal right adrenalectomy. Asvide 2019;6:197. Available online: http://www.asvide.com/article/view/32876
- Raffaelli M, De Crea C, Bellantone R. Real time laparoscopic lateral transabdominal left adrenalectomy. Asvide 2019;6:198. Available online: http://www.asvide.com/article/view/32880
- Lombardi CP, Raffaelli M, De Crea C, et al. Endoscopic adrenalectomy: is there an optimal operative approach? Results of a single-center case-control study. Surgery 2008;144:1008-14. [Crossref] [PubMed]
- Henry JF, Sebag F, Iacobone M, et al. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World J Surg 2002;26:1043-7. [Crossref] [PubMed]
- Lombardi CP, Raffaelli M, De Crea C, et al. Role of laparoscopy in the management of adrenal malignancies. J Surg Oncol 2006;94:128-31. [Crossref] [PubMed]
- Carnaille B. Adrenocortical carcinoma: which surgical approach? Langenbecks Arch Surg 2012;397:195-9. [Crossref] [PubMed]
- MacGillivray DC, Whalen GF, Malchoff CD, et al. Laparoscopic resection of large adrenal tumors. Ann Surg Oncol 2002;9:480-5. [Crossref] [PubMed]
- Suzuki K. Adrenal laparoscopic surgery for malignant adrenal tumors. Biomed Pharmacother 2002;56 Suppl 1:139s-144s. [Crossref] [PubMed]
- Moinzadeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol 2005;173:519-25. [Crossref] [PubMed]
- Porpiglia F, Fiori C, Tarabuzzi R, et al. Is laparoscopic adrenalectomy feasible for adrenocortical carcinoma or metastasis? BJU Int 2004;94:1026-9. [Crossref] [PubMed]
- Fassnacht M, Allolio B. What is the best approach to an apparently nonmetastatic adrenocortical carcinoma? Clin Endocrinol (Oxf) 2010;73:561-5. [Crossref] [PubMed]
- Soon PS, Yeh MW, Delbridge LW, et al. Laparoscopic surgery is safe for large adrenal lesions. Eur J Surg Oncol 2008;34:67-70. [Crossref] [PubMed]
- Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg 2001;25:905-13. [Crossref] [PubMed]
- Bellantone R, Lombardi CP, Raffaelli M. What is the appropriate role of minimally invasive vs. open surgery for small adrenocortical cancers? Curr Opin Oncol 2015;27:44-9. [Crossref] [PubMed]
- Miller BS, Doherty GM. Surgical management of adrenocortical tumours. Nat Rev Endocrinol 2014;10:282-92. [Crossref] [PubMed]
- Icard P, Chapuis Y, Andreassian B, et al. Adrenocortical carcinoma in surgically treated patients: a retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery 1992;112:972-9. [PubMed]
- Crucitti F, Bellantone R, Ferrante A, et al. The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstitutional series of 129 patients. Surgery 1996;119:161-70. [Crossref] [PubMed]
- Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab 2013;98:4551-64. [Crossref] [PubMed]
- Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282-326. [Crossref] [PubMed]
- Ronchi CL, Kroiss M, Sbiera S. Current and evolving treatment options in adrenocortical carcinoma: where do we stand and where do we want to go? Eur J Endocrinol 2014;171:R1-11. [Crossref] [PubMed]
- Gaujoux S, Mihai R. joint working group of ESES and ENSAT. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 2017;104:358-76. [Crossref] [PubMed]
- Miccoli P, Raffaelli M, Berti P, et al. Adrenal surgery before and after the introduction of laparoscopic adrenalectomy. Br J Surg 2002;89:779-82. [Crossref] [PubMed]
- Miller BS, Ammori JB, Gauger PG, et al. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg 2010;34:1380-5. [Crossref] [PubMed]
- O’Neill CJ, Spence A, Logan B, et al. Adrenal incidentalomas: risk of adrenocortical carcinoma and clinical outcomes. J Surg Oncol 2010;102:450-3. [Crossref] [PubMed]
- Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 2009;115:243-50. [Crossref] [PubMed]
- Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 2010;17:263-70. [Crossref] [PubMed]
- Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery 2005;138:1078-85. [Crossref] [PubMed]
- Leboulleux S, Deandreis D, Al Ghuzlan A, et al. Adrenocortical carcinoma: is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol 2010;162:1147-53. [Crossref] [PubMed]
- Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer 2005;12:667-80. [Crossref] [PubMed]
- Porpiglia F, Fiori C, Daffara F, et al. Retrospective evaluation of the outcome of open versus laparoscopic adrenalectomy for stage I and II adrenocortical cancer. Eur Urol 2010;57:873-8. [Crossref] [PubMed]
- Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol 2010;58:609-15. [Crossref] [PubMed]
- Miller BS, Gauger PG, Hammer GD, et al. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery 2012;152:1150-7. [Crossref] [PubMed]
- Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2014;99:455-61. [Crossref] [PubMed]
- Cooper AB, Habra MA, Grubbs EG, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc 2013;27:4026-32. [Crossref] [PubMed]
- Mir MC, Klink JC, Guillotreau J, et al. Comparative outcomes of laparoscopic and open adrenalectomy for adrenocortical carcinoma: Single, high-volume center experience. Ann Surg Oncol 2013;20:1456-61. [Crossref] [PubMed]
- Lombardi CP, Raffaelli M, De Crea C, et al. Open vs endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery 2012;152:1158-64. [Crossref] [PubMed]
- Donatini G, Caiazzo R, Do Cao C, et al. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): a retrospective comparative cohort study of laparoscopic versus open approach. Ann Surg Oncol 2014;21:284-91. [Crossref] [PubMed]
- Fosså A, Røsok BI, Kazaryan AM, et al. Laparoscopic versus open surgery in stage I-III adrenocortical carcinoma: a retrospective comparison of 32 patients. Acta Oncol 2013;52:1771-7. [Crossref] [PubMed]
- Anderson KL Jr, Thomas SM, Adam MA, et al. Each procedure matters: threshold for surgeon volume to minimize complications and decrease cost associated with adrenalectomy. Surgery 2018;163:157-64. [Crossref] [PubMed]
- Park HS, Roman SA, Sosa JA. Outcomes from 3144 adrenalectomies in the United States: which matters more, surgeon volume or specialty? Arch Surg 2009;144:1060-7. [Crossref] [PubMed]
- Lombardi CP, Raffaelli M, Boniardi M, et al. Adrenocortical carcinoma: effect of hospital volume on patient outcome. Langenbecks Arch Surg 2012;397:201-7. [Crossref] [PubMed]
- Bergamini C, Martellucci J, Tozzi F, et al. Complications in laparoscopic adrenalectomy: the value of experience. Surg Endosc 2011;25:3845-51. [Crossref] [PubMed]
- Hauch A, Al-Qurayshi Z, Kandil E. Factors associated with higher risk of complications after adrenal surgery. Ann Surg Oncol 2015;22:103-10. [Crossref] [PubMed]
- Palazzo F, Dickinson A, Phillips B, et al. Adrenal surgery in England: better outcomes in high-volume practices. Clin Endocrinol (Oxf) 2016;85:17-20. [Crossref] [PubMed]
- Gallagher SF, Wahi M, Haines KL, et al. Trends in adrenalectomy rates, indications, and physician volume: a statewide analysis of 1816 adrenalectomies. Surgery 2007;142:1011-21; discussion 1021. [Crossref] [PubMed]
- Murphy MM, Witkowski ER, Ng SC, et al. Trends in adrenalectomy: a recent national review. Surg Endosc 2010;24:2518-26. [Crossref] [PubMed]
- Villar del Moral JM, Rodríguez González JM, Moreno Llorente P, et al. Adrenal surgery in Spain: final results of a national survey. Cir Esp 2011;89:663-9. [Crossref] [PubMed]
- Gaujoux S, Bonnet S, Leconte M, et al. Risk factors for conversion and complications after unilateral laparoscopic adrenalectomy. Br J Surg 2011;98:1392-9. [Crossref] [PubMed]
- Bittner JG, Gershuni VM, Matthews BD, et al. Risk factors affecting operative approach, conversion, and morbidity for adrenalectomy: a single-institution series of 402 patients. Surg Endosc 2013;27:2342-50. [Crossref] [PubMed]
- Coste T, Caiazzo R, Torres F, et al. Laparoscopic adrenalectomy by transabdominal lateral approach: 20 years of experience. Surg Endosc 2017;31:2743-51. [Crossref] [PubMed]
- Thompson LH, Nordenström E, Almquist M, et al. Risk factors for complications after adrenalectomy: results from a comprehensive national database. Langenbecks Arch Surg 2017;402:315-22. [Crossref] [PubMed]
- Chen Y, Scholten A, Chomsky-Higgins K, et al. Risk Factors Associated With Perioperative Complications and Prolonged Length of Stay After Laparoscopic Adrenalectomy. JAMA Surg 2018;153:1036-41. [Crossref] [PubMed]
- Kazaure HS, Roman SA, Sosa JA. Obesity is a predictor of morbidity in 1,629 patients who underwent adrenalectomy. World J Surg 2011;35:1287-95. [Crossref] [PubMed]
- Seifman BD, Dunn RL, Wolf JS. Transperitoneal laparoscopy into the previously operated abdomen: effect on operative time, length of stay and complications. J Urol 2003;169:36-40. [Crossref] [PubMed]
- Castillo OA, Vitagliano G, Secin FP, et al. Laparoscopic adrenalectomy for adrenal masses: does size matter? Urology 2008;71:1138-41. [Crossref] [PubMed]