Open adrenalectomy
Historical notes
Before the introduction of laparoscopic adrenalectomy in early 1990s, open adrenalectomy was the only surgical option. Surgery of the adrenal glands emerged as part of abdominal surgery at the end of the 19th century. In 1889, Knowsley-Thornton reported the removal of a large adrenal tumour and in 1926, Roux in Lausanne, Switzerland, and Charles Mayo in Rochester, Minnesota, successfully removed a phaeochromocytoma (1).
The anterior approach was initially advocated by Cahill, one of the pioneers of adrenal surgery (2). The posterior approach was originally described by Young (3) and offered the technical advantage of being extraperitoneal, extrapleural, and subdiaphragmatic and the clinical advantage of being associated with low postoperative morbidity. In current day surgical practice the posterior approach has become obsolete, as all patients who in the past were deemed to benefit from this procedure are currently being offered laparoscopic or retroperitoneoscopic adrenalectomy.
This chapter will focus on the technical aspects of open adrenalectomy. More detailed discussion of the assessment and management of patients with adrenocortical cancer (ACC) have been summarised in the recent guidelines written by the European Society of Endocrinology (4) and the perioperative care has been discussed in guidelines written by the European Society of Endocrine Surgeons (ESES) and the European Network for Study of Adrenal Tumours (ENSAT) (5). Issues related to training in adrenal surgery and the need to centralise such operations in centers with a defined annual workload will be addressed I the upcoming meeting of the European Society of Endocrine Surgeons and will be published later this year.
Indication for open adrenalectomy
Even though minimally invasive techniques for adrenalectomy have been adopted in many surgical centres, there is a need to be, remain or become confident with open adrenalectomy. Patients with large adrenocortical tumours (>6–8 cm) and those with CT suspicion of locally invasive tumours expected to have an adrenocortical cancer should have an open operation. In addition, laparoscopic adrenalectomy should be converted to open operation in case there is macroscopic appearance suspicious of malignancy (invasion in surrounding structures, presence of regional lymphadenopathy) or if the surgeon is concerned that the tumour could not be removed without avoiding tumour fragmentation/spillage. Conversion to open operation might also prove necessary if intraoperative incidents (e.g., uncontrolled bleeding) cannot be managed laparoscopically.
Adrenal tumours with extension into major venous structures should all be approached through an open operation.
Large phaeochromocytomas of up to 8–10 cm might still be approached laparoscopically by surgeons with appropriate experience but the larger the diameter of such tumours the more likely is that open adrenalectomy will be necessary.
Bilateral adrenalectomy is not an indication for open approach as laparoscopic or retroperitoneoscopic surgery is feasible in these patients [discussed in (6)].
Previous abdominal surgery is not a strict contraindication for laparoscopic surgery. Though some of these patients might benefit from retroperitoneoscopic approach (i.e., avoiding the possible need to deal with adhesions after previous surgery), laparoscopic approach is feasible in the vast majority of them.
Who should perform open adrenalectomy?
The current provision of adrenal surgery is inadequate. Analysis of Hospital Episodes Statistics showed that in the United Kingdom over 200 surgeons are performing adrenal surgery of whom only 34 surgeons performed more than 6 cases per year and 189 surgeons had a median number 1 adrenalectomy/year (7). Knowing that the 2017 report national audit kept by the British Association of Endocrine and Thyroid Surgeons recorded 331 open adrenalectomies and 1,555 laparoscopic adrenalectomies one can extrapolate that 1 in every 5 adrenalectomies are open and that the vast majority of surgeons involved in this type of surgery would perform one single such cases every few years. This situation is untenable as it compromises the care of the subgroup of patients with most aggressive adrenal tumours. The need for change is imperative and drives the ongoing efforts to centralise adrenal surgery in the UK. The situation is likely to be similar in most other countries. It is expected that the 2019 meeting of the European Society of Endocrine Surgeons focused on volume-outcome correlations will formulate guidance for establishing centres of excellence in adrenal surgery and this would help patients and referring clinicians make more informed choices.
During the process of writing the joint guidelines from ESES and ENSAT for the surgical care of patients with adrenocortical cancer the author collected information from surgeons involved in the working group (listed in Acknowledgements). Data on 123 patients operated in 13 centres over 5 years was analysed and formed the basis of many of the comments made in this paper.
Informed consent
The preoperative imaging facilitates staging of the disease and influences the extent of the planned operation. For patients with small-volume/localised disease, excision of tumour and perinephric fat and local lymph nodes is deemed beneficial and can be achieved with minimal morbidity hence the consent will focus mainly on generic risks associated with extensive abdominal surgery (ileus, chyle leak, deep vein thrombosis, respiratory difficulties). Patients with locally advanced disease should be informed about the possibility of ipsilateral nephrectomy, splenectomy and/or distal pancreatectomy and the consent process should include information about the risks associated with each of these additional procedures (e.g., decrease of renal function, pancreatic leak, post-splenectomy sepsis).
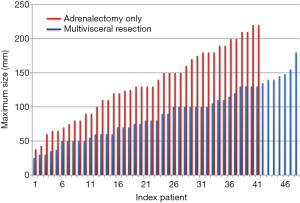
In our analysis, the use of multivisceral resection was not strictly dependent on the size of the primary tumours (Figure 1). Out of 101 patients who had open adrenalectomy for ACC, adrenalectomy-only was performed in 49 patients while the other had also nephrectomy (n=37), splenectomy (n=12), pancreatectomy (n=9) or liver resection (n=12).
Whether or not the need for such multivisceral resections is apparent preoperatively, the informed consent has to include all these possible procedures as each can trigger a specific set of complications. This issue has become increasingly significant after the change in the principle guiding the consent process from the Bolam test (‘what will be done by the majority of clinicians in a similar situation’) to the Montgomery rule (‘everything that can have a serious impact on the life of the patient, irrespective of how small is the risk’). In this context it has become increasingly important to allow patients to choose from the range of options, including avoidance of surgery. Therefore the decision to offer surgery to patients with metastatic disease or when a complete (R0) resection is unlikely to be achieved should be discussed in a multidisciplinary meeting, with input from clinicians with previous experience with the management of such cases. The threshold for referral to a tertiary centre should be very low unless the local surgical/medical/oncological teams have accumulated already significant experience with the management of ACC.
For patients with phaeochromocytomas, the consent process should include details of the preoperative adrenergic blockade. Based on local practice, a preoperative meeting with the anaesthetist involved in the operation is very important for establishing the appropriate dose of medication. Though this is routine practice in our unit, other centers are moving away from using adrenergic blockade in all patients and some might not include the anaesthetist in the preoperative preparation of patients.
Perioperative management
Deep vein thrombosis prophylaxis is provided by intraoperative use of flowtron pumps followed by postoperative TEDs (anti-embolism stockings) and subcutaneous low molecular weight heparin (Clexane/Daltaparin/Fragmin, according to local protocol).
Antibiotic prophylaxis is routinely used in patients with Cushing syndrome but can be omitted in patients with non-secreting cortical tumours or phaeochromocytomas. If splenectomy is anticipated as part of the procedure patients should be considered for preoperative vaccination. Alternatively post-splenectomy prophylaxis with Penicillin V is started immediately postoperative period and vaccination can be completed 3–4 weeks later.
Intravenous steroids should be given intraoperatively (100 mg Hydrocortisone on induction) in patients with Cushing syndrome.
Adrenergic blockade for patients with phaeochromocytomas is used in vast majority of patients and is decided based on local experience/availabilities.
Position on table
In our practice patient is supine, with a wedge placed on the side operated on. More pronounced lateral decubitus can be considered based on personal references.
Choice of incision
It is the author’s preference to use a bilateral subcostal incision (‘roof top’) with a possible midline vertical extension. Some reported the use of a thoracoabdominal incision to allow better access to the upper pole of large tumours or in cases when there is radiological evidence of diaphragmatic invasion.
Surgical technique for right open adrenalectomy for ACC
- After initial general inspection of the abdominal cavity, the operation starts by mobilising the colonic hepatic flexure by dividing the lateral part of the gastrocolic ligament and the peritoneal reflection over the ascending colon. In order to achieve full access to inferior vena cava (IVC) the duodenum is Kocherised.
- Mobilising the liver. In order to facilitate the dissection of the tumour at a later stage in the operation, the ‘mobility’ of the liver has to be increased by dividing the falciform ligament and the lateral triangular ligament. In our practice, at this stage of the operation the Thompson retractor is secured in position so that the ribs can be lifted and better access be secured.
- Mobilising the right kidney. For small adrenal tumours that can be easily dissected off the upper pole of the right kidney the Gerota fascia is opened towards the upper pole of the kidney and the perinephric fat mobilised upwards so that it becomes part of the future surgical specimen. For larger adrenal tumours that overlap renal vessels should be removed en bloc with the right kidney. In such cases, the dissection of retroperitoneal space starts at the lower pole of the kidney from lateral to medial. The right ureter is identified, tied and divided. The right gonadal vessels should be identified and protected up to their drainage point into the IVC. The renal vessels are tied and divided. A sling should be passed around the IVC and the left renal vein if it is expected to need to clamp the IVC later in the procedure.
- Mobilising the liver. If there is evidence of IVC invasion on preoperative CT scan or if the tumour is densely adherent to IVC and the right adrenal vein cannot be safely demonstrated, one needs to secure control of IVC at subdiaphragmatic level. The liver is fully mobilised off the diaphragm until de suprahepatic veins are demonstrated and the left triangular ligament is divided so that access to suprahepatic IVC is secured. Careful dissection close to the crus of the diaphragm allows the IVC to be prepared for later clamping, if needed. In such cases it is routine practice to get control of distal IVC just distal to the insertion of the right renal vein and to sling the left renal vein.
- Dissection off the IVC. From the infrarenal IVC exposed earlier the dissection progresses proximally aiming to create a “groove” between the tumour and the IVC. Care should be shown close to small veins draining the caudate lobe of the liver into the IVC—ligation and division of these veins allows further upwards mobilisation of the liver.
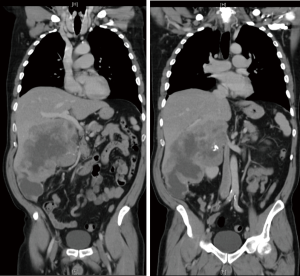
- Dissection off the right lobe of the liver. One needs to assess if there is a dissection plane that would allow mobilisation of the tumour without breaching its capsule. If there is direct invasion into the liver, one has to ask support from a liver surgeon who could assist in performing a limited right hepatectomy in continuity with the tumour. This emphasis the need for careful preoperative multidisciplinary input and the need to centralise such cases in centres where appropriate multidisciplinary expertise exists.
Perioperative findings of such a case are presented in Figure 2.
Surgical technique for left open adrenalectomy for ACC
- Mobilising the left colon. The splenic flexure is mobilised by dividing the gastrocolic ligament and the peritoneal reflection along the descending colon so that the left colon mobilised distally and towards the midline until the fourth part of duodenum becomes visible (Treitz angle).
- Management of the spleen. If the splenic artery is seen on preoperative CT scans to be surrounded or displaced by the tumour, it is safer (and easier) to perform a simultaneous splenectomy. This also allows easier access to subcostal space as in patients with very large adrenal tumours it might be impossible to dissect the upper pole of the tumour off the diaphragm if the spleen has not been removed earlier in the operation.
Identifying the splenic artery at the upper border of the pancreas early during the dissection allows control of the main arterial splenic inflow and might minimise blood loss later during en bloc resection. The gastrosplenic arteries are divided individually with attention to preserving the anastomotic vessels along the great curvature of the stomach within the gastrosplenic ligament. Direct invasion of the adrenal tumour into the stomach has not been encountered in any of the ACC cases reported by the ESES working group.
- Mobilising the left kidney. The retroperitoneal space is dissected from lateral to medial, starting at the lower pole of the kidney. The left ureter is identified, tied and divided. The gonadal vessels should be identified, tied and divided distal from their drainage point into left renal vein. Soft tissue along the IVC is mobilised en bloc as it is likely to contain the regional lymph nodes.
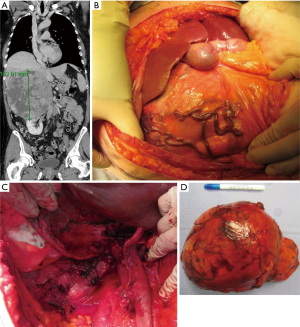
- Management of the pancreas. In the presence of a large left adrenal tumour, the tail of the pancreas is “stretched” over the tumour and a distal pancreatectomy might provide a safer oncological procedure. This can be avoided in many cases as the ACC rarely (if ever) invades directly into the pancreas. When deemed beneficial to perform distal pancreatectomy, a tunnel is created under the body of the pancreas to the left of inferior mesenteric vein and the body of the pancreas is transected using a linear stapler. The resection line is usually sutured. A Robinson drain is left next to the pancreatic bed as a pancreatic leak is a common postoperative complication.
Perioperative findings of such a case are presented in Figure 3.
Role of lymphadenectomy
Lymph node dissection (LND) is yet to become a formal component of radical adrenalectomy. Because the lymphatic drainage of the adrenal gland includes the renal hilum lymph nodes, and the para-aortic and paracaval lymph nodes, it is expected that many of these lymph nodes are included if en bloc resection of the ACC is performed to include the kidney, perinephric fat and Gerota’s fascia.
The wide range of reported lymph node involvement in ACC (from 5% to 75%) suggests that formal regional lymphadenectomy is neither formally performed by surgeons nor accurately assessed or reported by pathologists. According to large American and French series, approximately one-third of patients with ACC had formal lymphadenectomy as part of the tumour resection, reflecting the heterogeneity of operative management. Similarly, the ESES-ENSAT working group declared that in their practice lymph nodes were ‘not seen/not dissected’ (n=21) or were ‘likely excised en bloc’ (n=5), excised en bloc (n=5) or dissected on purpose (n=11) (Figure 4). It is the authors’ experience that macroscopic identification of such lymph nodes is very challenging and the en-bloc resection of the soft tissue surrounding the large vessels is commonly associated with postoperative chyle leak. In our experience we attempted to use indocyanine green to identify periadrenal lymph nodes (similar to the protocol introduced in colonic resection) but failed to visualise convincing uptake of the dye in local lymph nodes (personal data, unpublished).
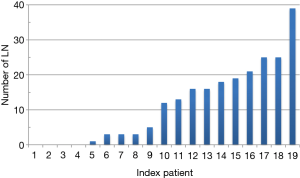
The guidelines published recently by the European Society of Endocrinology state: ‘The panel suggests that routine locoregional lymphadenectomy should be performed with adrenalectomy for highly suspected or proven ACC. It should include (as a minimum) the periadrenal and renal hilum nodes. All suspicious or enlarged lymph nodes identified on preoperative imaging should be removed’ (4).
A prospective multicentre cohort study would be invaluable to address the issue of feasibility of lymphadenectomy for ACC and to quantify its benefits (staging) and associated added morbidity.
En bloc multivisceral resections
A retrospective study compared the oncological results of patients with stage II ACC treated by radical adrenalectomy alone (n=16) or by nephron-adrenalectomy (n=25). The results did not support the hypothesis that nephrectomy improves the oncological outcome (8). In a multicenter European study on surgery for ACC, pathological invasion of the kidney was observed in only 30% of the patients with combined nephrectomy. Combined nephrectomy, however, offers a lower risk of tumour capsular rupture and can facilitate complete lymphadenectomy of the renal hilum. The ESE guidelines state: ‘The panel recommends that adjacent organs should be resected en bloc if they are suspected to be invaded. This includes the spleen, distal pancreas, stomach, kidney, right liver, colon, diaphragm, and the wall of the IVC or left renal vein. No data to compare outcomes but it is deemed to be ‘good surgical practice’. The panel suggests that in the absence of direct renal invasion routine resection of the ipsilateral kidney should be avoided’ (4).
Surgery for ACC with venous tumour thrombus
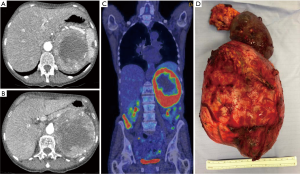
Extension of ACC to the adrenal, renal vein or IVC occurs in approximately 25%. Venous involvement consists mostly of intravenous tumour thrombus, but can be associated with direct vascular invasion. Thrombectomy may require IVC cross-clamping above or below the hepatic vein confluence or cardiopulmonary bypass, depending on the upper level extent of the thrombus. The resection should include complete thrombectomy, a flush manoeuvre and, occasionally, vascular cuff or prosthetic IVC replacement. A 3-year overall survival rate of 25–29 per cent in a large series encourages the performance of a venous resection in the presence of IVC or renal vein invasion (9). The ESE guidelines state: ‘The panel recommends that individualised treatment decision have to be made for such patients based on multidisciplinary input from endocrine surgeons, liver surgeons, cardiac/vascular surgeons. Such patients should not be declared as ‘unresectable’ until review in a regional centre where adequate expertise exists’ (4).
The role of multidisciplinary input cannot be overestimated in this context. When extensive vascular involvement or tumour extent around portal vessels is encountered a decision to not operate is mandatory (Figure 5).
Postoperative care
DVT prophylaxis continues during the admission, in parallel with early mobilisation. Oral intake can be resumed within 24 hours postoperatively.
Steroid replacement: for patients with Cushing syndrome intravenous steroids (100 mg hydrocortisone iv tds or qds) are maintained until diet is restarted and then converted to oral steroids (hydrocortisone, 20–20–10 mg/day, aiming to decrease by 5 mg/day every 3–5 days). Involvement of the endocrinology team is important for monitoring of long-term steroid replacement.
Summary comments
In an era when minimally invasive adrenalectomy is the gold standard treatment for majority of patients presenting with adrenal tumours, open adrenalectomy has become an operation that should be centralised in regional referral centers. The need for preoperative and postoperative multidisciplinary input and the technical challenges of the operation should convince most surgeons to refer such cases to recognised centres with previous experience in the management of these patients.
Acknowledgments
The author is grateful for the following members of the ESES-ENSAT working group for sharing details of their personal experience in the management of ACC: Bruno Carnaille (France); Betrand Dousset (France); Frédéric Dumont (France); Cristian Fiori (Italy); Sebastien Gaujoux (France); Jean-Louis Kraimps (France); Hans Langenhuijsen (Netherlands); Kerstin Lorenz (Germany); Muriel Mathonnet (France); Eric Mirallié (France); Els J. M. Nieveen van Dijkum (Netherlands); Marco Raffaeli (Italy); Nada Rayes (Germany); Frédéric Sébag (France); Frédéric Triponez (Switzerland); Andrea Valeri (Italy); Franck Zinzindohoue (France).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Welbourn B. The History of Endocrine Surgery. New York: Praeger, 1990:147.
- Cahill GF. Hormonal tumours of the adrenals. Surgery 1944;16:233.
- Young HH. Genital abnormalities: Hermaphroditism and related adrenal disease. Baltimore: Williams & Wilkins, 1937.
- Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2018;179:G1-G46. [Crossref] [PubMed]
- Gaujoux S, Mihai R. joint working group of ESES and ENSAT. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 2017;104:358-76. [Crossref] [PubMed]
- Maccora D, Walls GV, Sadler GP, et al. Bilateral adrenalectomy: a review of 10 years' experience. Ann R Coll Surg Engl 2017;99:119-22. [Crossref] [PubMed]
- Palazzo F, Dickinson A, Phillips B, et al. Adrenal surgery in England: better outcomes in high-volume practices. Clin Endocrinol (Oxf) 2016;85:17-20. [Crossref] [PubMed]
- Porpiglia F, Fiori C, Daffara FC, et al. Does nephrectomy during radical adrenalectomy for stage II adrenocortical cancer affect patient outcome? J Endocrinol Invest 2016;39:465-71. [Crossref] [PubMed]
- Mihai R, Iacobone M, Makay O, et al. Outcome of operation in patients with adrenocortical cancer invading the inferior vena cava--a European Society of Endocrine Surgeons (ESES) survey. Langenbecks Arch Surg 2012;397:225-31. [Crossref] [PubMed]