
Groove pancreatitis: a challenging imaging diagnosis
Introduction
Groove pancreatitis (GP) is an under-recognized form of chronic pancreatitis (CP) that involves the space between the pancreatic head, the duodenum, and the common bile duct (CBD). First described by Becker in 1973 with the German word ‘Rinnenpankreatitis’ (1) to describe the segmental pancreatitis in the groove area. Stolte et al. (2) coined the term “groove pancreatitis” and classified it into two forms: a pure form in which the fibro-inflammatory changes affect exclusively the pancreatic-duodenal groove (the space between the pancreas head, the duodenum and the CBD) and a segmental extending medially from the pancreatic-duodenal groove into the pancreatic head. These two forms were found to account for 8.9% and 15.5%, respectively, of 123 pancreaticoduodenectomies performed on patients with CP (2). In the pure form, in most cases the parenchyma and main pancreatic duct (MPD) are not involved while in the “segmental” form, the scarring tissue affects the dorso-cranial portion of the pancreatic head involving the MPD with a CP in addition to groove involvement (3).
Other terms used to describe GP have been used including para-duodenal pancreatitis, duodenal cystic dystrophy, duodenal heterotopic pancreas and pancreatic hamartoma of the duodenum. Subsequently Adsay and Zamboni (4) have grouped GP, with cystic dystrophy of the pancreas, pancreatic hamartoma of duodenum, paraduodenal wall cyst and myoadenomatosis, and proposed a universal name referred to as “para-duodenal pancreatitis”.
The separation between the pure and segmental form of GP is often confounded because in some cases patients with initial pure GP may show mild, regular and progressive narrowing of the pancreatic duct with consequent evolution into CP (5).
Even in the most specialized centers, many untrained radiologists may encounter difficulty to make the proper diagnosis. Nowadays, given the lesser application of surgical treatment of GP, it becomes more problematic to accurately establish an estimate of the prevalence of the disease. GP can be mistaken for carcinoma, can coexist with it or mask its presence (2,6-11).
The purpose of this review is to raise radiologists’ knowledge in etiology, pathogenesis, clinical manifestations and radiologic appearance of GP showing the imaging appearance of GP at computed tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP).
Clinical presentation
Most patients with GP are male, aged 40–50 years, with a history of severe chronic alcoholism and, in a lower percentage, also associated with smoking (12).
Abdominal pain, postprandial vomiting and weight loss, primarily due to duodenal obstruction, are the most common manifestations of GP, with concomitant twofold or threefold increase of serum amylase concentration, or heavy levels of serum lipase (4).
About 80% of the patients with the clinical symptoms of acute pancreatitis present such high concentrations of the serum of pancreatic amylase and serum lipase levels. Tumor markers (CA 19-9 and CEA) are usually normal (13,14).
It has also been reported that diarrhea or diabetes mellitus are commonly associated with GP (15). The clinical symptoms of the syndrome spread over the time span ranging from a few weeks to a year, then the GP becomes chronic (16). In a high number of patients with alcohol abuse, obstructive jaundice has been observed especially in the course of chronic disease if late stenosis of the CBD has occurred (17).
Etiology and pathogenesis
The etiology of GP is likely heterogeneous implying a series of factors possibly playing a role in its development. There is general agreement about the effects for people who abuse of ethyl alcohol on disease development and all its clinical manifestations (18).
Chronic alcohol intake causes a decrease in bicarbonate secretion which increases viscosity and consequent stagnation of pancreatic secretion in pancreatic ducts; it follows an increase in pressure inside the Santorini duct with the release of the secretion in the groove that promotes the formation of pseudocysts (19).
One of the mechanisms hypothesized for the development of CP associated with the alcohol abuse provides that alcohol predispose acinar cells to autodigestive injury and necro-inflammation by increasing the synthesis of digestive and lysosomal enzymes leading to autodigestive cellular damage, acinar injury and pancreatic necro-inflammation.
It is now accepted that the disease progresses irreversibly from the initial stages of necro-inflammation towards chronic stage of acute pancreatic through repeated attack episodes (20-22). The latter produce additional and permanent structural damage to the gland, in the segmental form, resulting in the changes of CP characterized histologically by acinar atrophy and fibrosis (the necrosis-fibrosis sequence), which impair both endocrine and exocrine pancreatic functions (23). Although not completely elucidated the mechanism(s) responsible for the development of pancreatic fibrosis, however, central in pancreatic fibrogenesis have been identified the stellate cells in a manner analogous to hepatic stellate cells (24-28).
Anatomical and functional predisposing causes
The pancreatic-duodenal groove is a “theoretic” space between the pancreatic head and the duodenal wall (Figure 1).

The characteristic location of GP in the region of Santorini duct, has suggested the presence of an anatomical or functional obstruction of the minor papilla, with consequent severe ductal inflammation.
Cystic dystrophy and heterotopic pancreas (CDHP) are a predisposing anatomical factor of CP, duodenal obstruction and obstructive jaundice, due to the presence of pancreatic tissue at the duodenal wall, reflecting the incomplete involution of the dorsal pancreas at this region (4).
Surgical procedures used in gastrectomy or also gastroduodenal ulcer, and biliary disease can alter normal anatomy contributing to the development of GP. A confirmation of the possibility of GP appearance as a result of anatomical disturbances is confirmed by the case reported for a 69-year-old patient with a history of multiple gastroduodenal ulcers, who, admitted for evaluation of abdominal pain, presented an obstructive jaundice (29). Patient underwent CT and MRI scans that showed typical sheet-like mass in the pancreaticoduodenal groove. Subsequently, the same patient underwent endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) whose biopsy results excluded the presence of tumor. In presence of persistent and acute pain, the patient underwent pancreatic-duodenectomy and the subsequent anatomopathological analysis demonstrated that the patient had inflammatory tissue in the pancreaticoduodenal groove associated with duodenal ulcer penetrating the pancreas.
The authors assumed that a spreading of inflammation combined with the duodenal ulcer might have been one of the causes of GP (29).
Macroscopic and microscopic histology
For a correct diagnosis of GP, it is important to examine the area at the junction of the pancreatic head and the descending duodenum near the minor papilla. In most cases it is possible to observe thickening and scarring tissue involving the duodenal wall that usually causes stenosis of the second portion of the duodenum, and may lead to CBD stricture (30).
Small cysts or even a multilocular cystic mass are often seen within the thickened wall or in the pancreaticoduodenal groove itself that compress the bile duct (4,14,31).
Some of these cysts contain clear fluid, but others may contain stones or granular, white material. When the cysts reach large dimensions (more than 10 cm) and are located in the pancreatic tract of the supra-ampullary duodenum, they form the so-called “para-duodenal wall cyst” mimicking intestinal duplication (4).
Intraduodenal cysts have been detected in about half of the patients with GP. Becker et al. (3) have identified intraduodenal cysts in 49% of patients with GP. Enlarged lymph nodes are seen usually around the pancreatic head.
At the early stage of GP pancreatic head undergoes enlarged owing the large amount of free fluid accumulated in the groove between the head of the pancreas and duodenal sweep.
GP in the early phase can cause an accumulation of large amounts of free fluid in and around the pancreas, often in the groove between the head of the pancreas and duodenal sweep. This enlargement leads, ultimately, to intrinsic inflammatory reaction and fibrosis involving adjacent pancreatic tissue and may lead to the dilation of the MPD.
If progressive the enlargement leads to inflammatory reaction and fibrosis. The fibrotic process involving adjacent pancreatic tissue may lead to the dilation of the MPD.
In about 50% of cases, the patients present duodenal stenosis and/or bile duct restraints (30), which could be a sign of high risk for pancreatic cancer.
At histology the most common finding in GP is duodenal Brunner’s gland hyperplasia of the duodenal mucosa which contributes to the thickening of the duodenal wall (4,17).
Moreover, myoid cells of the duodenal wall underwent morphological changes near the minor papilla and exuberant proliferation creating a total of an image reminiscent of the “myo-adenomatosis”, mimicking pancreatic adenocarcinoma (32).
Some of the cysts were enclosed in layers of a smooth muscle and areas of dense collagenous stroma containing abundant fibroblasts (4,33,34).
Microscopic examination reveals, as mentioned above, that heterotopic pancreatic tissue occurs in both the submucosa or muscularis propria of the duodenal wall (35-38).
CT
The classic MDCT imaging features consist of loss of fat planes between head of pancreas and the duodenum with an ill-defined crescentic frank soft tissue mass seen with the pure form of GP.
The early phase of contrast-enhanced dynamic CT shows an ipodense area as consequence of considerably widened underlying fibrosis (Figure 2).
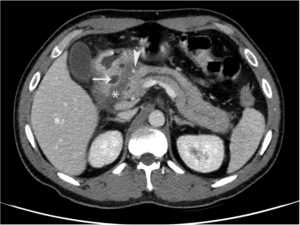
In the late phase of dynamic CT, the fibrotic tissue shows a delayed enhancement (39). The soft tissue appears as “sheet-like” curvilinear crescentic shape better evaluated on coronal multiplanar reformatted images (40).
Coronal reformatted images of a contrast enhanced CT of the medial duodenal wall reveals better large thickening; small cysts or even a multilocular cystic mass are often seen within the duodenal thickened wall or in the pancreatic-duodenal groove itself (41,42).
GP in the pure form can be unreliably differentiated from the segmental form of GP by MDCT imaging. The segmental form of GP exhibits a focal hypodense lesion in the pancreatic head in close proximity to the duodenal wall. The MPD may exhibit mild upstream dilatation in the pancreatic body and tail, while in the pure form of the disease the pancreas appears normal (43).
The CBD narrowing in the distal part may play a role in the development of upstream biliary dilatation. In both forms this narrowing is smooth, regular and tapered (Figure 3).

Pancreatic ductal irregularities have been described as particularly common in GP cases (especially in its segmental form), as the pancreatic head is directly involved by extensive fibrosis (3). However, ductal dilatation can be detected, even in the “pure form” of GP, subsequent to extrinsic compression of the pancreatic head caused by the groove mass. Even in cases of extensive disease, peripancreatic vessels are usually maintained, without thrombosis signs or infiltration (44,45).
These CT signs and a medical history of alcohol abuse are highly suggestive of GP. In a case report (46), the patient, having a 10-year history of alcohol abuse, and history of recurrent acute pancreatitis was examined by CT. Imaging revealed bulky head of pancreas with a hypodense area between pancreatic head and second part of duodenum, prominent pancreatic duct, dilated CBD (15 mm) and stenosis of second part of duodenum, sufficient to reliably diagnose GP.
MRI
On MRI imaging, GP is characterized by sheet-like mass between the pancreatic head and the duodenum. The mass is hypointense to pancreatic parenchyma on T1-weighted images, and according to the time of disease onset can be hypo-, iso- or slightly hyperintense on T2-weighted images (5). Appropriate interpretation of the T2 sequences is useful to infer the degree of disease activity (14), because of the signal changes from hyperintense, in the initial phases, to iso-hypointense in the advanced phases (8), the variations registered being due to the progressive accumulation of fibrous connective tissues. In other terms, the subacute form of GP shows higher signal on T2-weighted images due to edema (Figure 4), while chronic form of the disease has a lower T2 signal due to fibrosis.

On delayed gadolinium-enhanced images, the diagnostic accuracy of MRI is comparable to that of CT in the characterization of fibrotic mass. Gadolinium-enhanced dynamic images show delayed enhancement, reflecting the fibrous nature of the tissue.
Ishigami et al. (47) reported in their study that 93% of the patients examined had a post-gadolinium patchy and late enhancement reflecting the inflammatory nature of pancreatic tissue.
Cystic lesions can also be well seen on T2-weighted images in the groove or duodenal wall (Figure 5) (5).

Cystic degeneration within the duodenal wall is a specific sign of GP. The typical findings are cysts, variable in size and complexity in the thickened duodenal wall.
MRCP has become an important diagnostic tool in a variety of pancreaticobiliary disorders and in particular in imaging workup of patients with GP.
Using MRCP, it is possible to detect the reduction of the caliber with regular smooth profile of the distal CBD. MRCP may show dilatation of the MPD in the form of segmental GP, while it may appear normal in the pure form. MRCP may show the dilation of the ampulla of Vater and the MPD, both of which are common gross features of segmental GP, detect relationships among cysts, CBD and MP, and also reveal the increase of the distance between duodenal lumen and distal duct (5).
Secretin-enhanced MRCP may supports the diagnosis and classification of CP, anomalies such as pancreas divisum, and santorinicele, which may cause impeded pancreatic outflow. Values for the sensitivity of secretin-enhanced MRCP were reported to vary in the range 73–100% and specificities of 97–100% (48); however, since no data are present in the literature regarding the secretin-enhanced-MRCP as a first choice procedure for the diagnosis of GP, its potential diagnostic role remains uncertain.
Compared to CT MRI better visualize the involvement of the pancreas since the head of the pancreas showed diffuse decreased T1 signal intensity as a result of parenchymal atrophy and fibrosis (49).
Duodenal wall thickening and mural cysts were also seen in all the patients examined by MRI in a series of 16 patients with GP (49). The medial wall of duodenum was involved in the pure as well as the segmental forms of GP, with multiple T2 hyperintense cysts in both the duodenal wall and PD groove. The authors found that MRCP enabled determination of relationship between cysts and CBD and pancreatic ducts (49).
Focal thickening and abnormal increased enhancement of the second part of the duodenum, and cystic changes in the region of the pancreatic accessory duct support the diagnosis of GP over pancreatic cancer with an accuracy of 87.2% and negative predictive value for cancer of 92.2% (50).
EUS and endoscopy-guided FNA biopsy
EUS represents one of the most sensitive methods for detecting pancreaticobiliary lesions. The potentialities of the EUS are multiple, as it can also detect thickening and stenosis of the second duodenal part with intramural cysts, smooth stenosis of the CBD; and in the segmental form, heterogeneous hypoechoic mass, enlargement of the pancreatic head, with calcifications or pseudocyst and dilatation of the MPD. It is generally accepted that with the use of EUS it is possible to localize the disease exactly and evaluate the surface involved, with the limitation being that EUS is not able to differentiate infiltration and inflammation. The possibility to obtain samples from suspicious lesions, by means of EUS-FNA as well as the use of contrast-enhanced, makes EUS an ideal modality for differentiate pancreatic adenocarcinoma from CP, allowing a cytohistological diagnosis in nearly 90% of cases (51). In fact endoscopy-guided FNA biopsy presents a great variability depending on the area sampled. If the sampled area has a plentiful hyperplasia localized to Brunner's glands, it can immediately be hypothesized that it corresponds to a neoplasm. Similarly, if any fibrotic area is discovered with the use of EUS, a neoplasm cannot be ruled out, as a desmoplastic reaction, mimicking an abnormal inflammatory alteration, is frequently associated with an adenocarcinoma (52-55).
Differential diagnosis and pitfalls
The differential diagnosis of GP includes pancreatic adenocarcinoma, periampullary cancers, pancreatic groove neuroendocrine tumor, cystic dystrophy of the duodenum and acute pancreatitis.
While the pure form is rather easy to identify, the segmental form of GP can be difficult to diagnose, because the groove involvement is often obscured by mass-like enlargement of the pancreatic head, so that GP can be easily confused with a pancreatic head mass (56,57). The most important differential diagnosis of GP, particularly in its segmental form, is adenocarcinoma of the head of the pancreas. The preoperative distinction between these entities has always been considered challenging (5). The findings usually associated with GP and not with neoplastic processes include: marked duodenal wall thickening and stenosis (5), cystic lesion of the groove area (54,58), smoothly tapered stricture of the intrapancreatic CBD, instead of an irregular and abrupt “shouldering” of the stricture that is found in case of pancreatic adenocarcinoma (8,57). This last sign serves to differentiate the GP from other conditions including duodenal cancer, ampulloma, and cholangiocarcinoma of the distal CBD (13,14). Contrast-enhanced MRI enables the radiologist to differentiate GP from pancreatic cancer if 3 signs are used as GP markers, i.e., the mural duodenal thickening, a delayed increase in the second part of the duodenum; and the presence of cysts inside the duodenal wall or the PD groove. These diagnostic parameters provided accurate diagnosis of GP reaching 87.2% and a negative predictive value of 92.9% in excluding cancer (50). Unlike most ductal adenocarcinomas of the pancreas, GP do not show the typical pancreatic double duct cutoff and upstream atrophy. Moreover, they tend to not infiltrate posteriorly into the retroperitoneum and not encase vasculature (5,54). Neuroendocrine tumors can be differentiated from GP by early hyper-enhancement due to their hypervascularity on postcontrast images, with peripheral ring-like enhancement on immediate post-gadolinium GRE, their hyperintensity on fat suppressed T2-weighted images and hypervascular liver metastases (59). GP differs from other forms of acute pancreatitis, associated with peripancreatic stranding and fluid, as they involve a large portion of the pancreatic parenchyma with inflammation tracking into the pararenal spaces. GP typically shows little retroperitoneal inflammation or fluid, and even in the segmental form, involvement of the pancreas is usually limited to the pancreatic head (60).
Discussion
The pancreatic-duodenal groove is a “theoretic” space between the pancreatic head and the duodenal wall. A number of small arteries, veins and lymphatic pass through this space. The most important vessel visible also or arterial phase of contrast enhanced imaging studies is the pancreatic-duodenal artery (PDA) that represent an important anatomical landmark (14). Each process arising medially respect to the PDA have a pancreatic origin. While each process arising laterally respect to the PDA have a duodenal or pancreatic-duodenal groove origin (Figure 6).

Moreover, many important anatomical structures are present in the pancreatic-duodenal groove space such as CBD, main and accessory pancreatic ducts, major and minor papilla. This anatomical complexity account for many of the clinical and imaging features of GP as well as for the differential diagnosis of this rare entity (14-19).
Indeed, many disorders centered in the groove should be considered as differential diagnosis when GP is suspected. Differential diagnosis of GP are mainly represented by pancreatic adenocarcinoma of the head of the pancreas, other pancreatic neoplasm, duodenal carcinoma, ampullary carcinomas, duodenal gastro intestinal stromal tumor (GIST) or duodenal neuro-endocrine tumor (NET), conventional acute edematous pancreatitis involving the groove (56,57).
Moreover, a number of different terms have been used to describe chronic inflammatory changes of the pancreatic-duodenal groove such as para-duodenal pancreatitis, duodenal heterotopic pancreas, duodenal pancreatic hamartoma, myoadenomatosis of the duodenal wall. All these entities described over time may be considered overall under the general entity of GP (1-19).
Moreover, GP may be centered into the groove (the pure form) or may extend into the pancreatic head (segmental form). Also in long standing pure form of GP chronic inflammation and fibrosis may lead to stricture of the CBD, duodenal stricture and/or obstructive chronic pancreatitis. From clinical point of view the association of GP with long standing ethanol assumption is the most relevant issue. Other clinical and biochemical findings may overlap with common form of CP and/or pancreatic adeno-carcinoma in case of biliary stricture (14).
Remarkably, fine needle biopsy of the duodenal wall by means of EUS are often non diagnostic (14). In fact, endoscopy-guided FNA biopsy presents a great variability depending on the area sampled. If the sampled area has a plentiful hyperplasia localized to Brunner’s glands, it can immediately be hypothesized that it corresponds to a neoplasm. Similarly, if any fibrotic area is discovered with the use of EUS, a neoplasm cannot be ruled out, as a desmoplastic reaction, mimicking an abnormal inflammatory alteration, is frequently associated with an adenocarcinoma (51-53).
Due to all these items the prospective diagnosis of GP and his differential diagnosis with pancreatic adenocarcinoma is still difficult and many patients undergo unnecessary Whipple procedure (56,57).
Moreover, in long standing GP with duodenal stricture, severe pain and pancreatic insufficiency Whipple procedure is indicated (41).
However is important to underlying some typical imaging features of GP that are uncommon in case of pancreatic adenocarcinoma that are represented by cystic changes into the duodenal groove and duodenal wall, thickening of the duodenal wall and delayed enhancement of the fibrotic tissue involving the duodenal-pancreatic groove (47) to suggest the diagnosis of GP and avoid unnecessary surgery in the early stage of disease.
Conclusions
GP is a rare form of CP centered into the pancreatic-duodenal groove more often encountered in middle-age ethanol-abuser. The recognition of imaging findings such as cystic changes of the pancreatic groove and duodenal wall thickening at CT and MRI and MRCP is important to suggest the diagnosis of groove pancreatitis. Unfortunately, the differentiation of GP only on the basis of imaging characteristics, clinical presentation and even with the aid of biological markers is very difficult, so that the patients often undergo pancreaticoduodenectomy (Whipple procedure) precisely because can be hard to completely exclude a neoplasm.
However, knowledge of the all the GP radiological features may address the radiologist towards the correct diagnosis exactly for the purpose of eliminating avoidable surgical interventions. In those cases where the imaging features are highly characteristic and the radiologist is able to strongly suggest the diagnosis on presentation, major surgery can potentially be avoided.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Becker V. Bauchspeicheldrüse. In: Doerr W, Seifert G, Uhlinger E. editors. Spezielle pathologische Anatomie. Vol 4. Berlin: Springer, 1973:252-445.
- Stolte M, Weiss W, Volkholz H, et al. A special form of segmental pancreatitis: groove pancreatitis. Hepatogastroenterology 1982;29:198-208. [PubMed]
- Becker V, Mischke U. Groove pancreatitis. Int J Pancreatol 1991;10:173-82. [PubMed]
- Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinicopathologically distinct entity unifying 'cystic dystrophy of heterotopic pancreas', 'paraduodenal wall cyst', and 'groove pancreatitis'. Semin Diagn Pathol 2004;21:247-54. [Crossref] [PubMed]
- Blasbalg R, Baroni RH, Costa DN, et al. MRI features of groove pancreatitis. AJR Am J Roentgenol 2007;189:73-80. [Crossref] [PubMed]
- Yamaguchi K, Tanaka M. Groove pancreatitis masquerading as pancreatic carcinoma. Am J Surg 1992;163:312-6. [Crossref] [PubMed]
- Irie H, Honda H, Kuroiwa T, et al. MRI of groove pancreatitis. J Comput Assist Tomogr 1998;22:651-5. [Crossref] [PubMed]
- Levenick JM, Gordon SR, Sutton JE, et al. A comprehensive, case-based review of groove pancreatitis. Pancreas 2009;38:e169-75. [Crossref] [PubMed]
- Manzelli A, Petrou A, Lazzaro A, et al. Groove pancreatitis. A mini-series report and review of literature. JOP 2011;12:230-3. [PubMed]
- Tan CH, Chow PK, Thng CH, et al. Pancreatic adenocarcinoma that mimics groove pancreatitis: Case report of a diagnostic dilemma. Dig Dis Sci 2006;51:1294-6. [Crossref] [PubMed]
- Malde DJ, Oliveira-Cunha M, Smith AM. Pancreatic carcinoma masquerading as groove pancreatitis: Case report and review of the literature. JOP 2011;12:598-602. [PubMed]
- Arora A, Rajesh S, Mukund A, et al. Clinicoradiological appraisal of ‘paraduodenal pancreatitis’: Pancreatitis outside the pancreas! Indian J Radiol Imaging 2015;25:303-14. [Crossref] [PubMed]
- Triantopoulou C, Dervenis C, Giannakou N, et al. Groove pancreatitis: a diagnostic challenge. Eur Radiol 2009;19:1736-43. [Crossref] [PubMed]
- Raman SP, Salaria SN, Hruban RH, et al. Groove pancreatitis: Spectrum of imaging findings and radiology-pathology correlation. AJR Am J Roentgenol 2013;201:W29-39. [Crossref] [PubMed]
- Rebours V, Lévy P, Vullierme MP, et al. Clinical and morphological features of duodenal cystic dystrophy in heterotopic pancreas. Am J Gastroenterol 2007;102:871-9. [Crossref] [PubMed]
- German V, Ekmektozoglou KA, Kyriakos N, et al. Pancreatitis of the gastroduodenal groove: a case report. Case Rep Med 2010;2010:329587.
- Pallisera-Lloveras A, Ramia-Ángel JM, Vicens-Arbona C, et al. Groove pancreatitis. Rev Esp Enferm Dig 2015;107:280-8. [PubMed]
- Balakrishnan V, Chatni S, Radhakrishnan L, et al. Groove pancreatitis: a case report and review of the literature. JOP 2007;8:592-7. [PubMed]
- Isayama H, Kawabe T, Komatsu Y, et al. Successful treatment of groove pancreatitis by endoscopic drainage via the minor papilla. Gastrointest Endosc 2005;61:175-8. [Crossref] [PubMed]
- Haber PS, Apte MV, Applegate TL, et al. Metabolism of ethanol by rat pancreatic acinar cells. J Lab Clin Med 1998;132:294-302. [Crossref] [PubMed]
- Zhang S, Wang C, Huang H, et al. Effects of alcohol drinking and smoking on pancreatic ductal adenocarcinoma mortality: A retrospective cohort study consisting of 1783 patients. Sci Rep 2017;7:9572. [Crossref] [PubMed]
- Apte M, Pirola R, Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid Redox Signal 2011;15:2711-22. [Crossref] [PubMed]
- Klöppel G, Maillet B. The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch A Pathol Anat Histopathol 1992;420:1-4. [Crossref] [PubMed]
- Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 2002;50:535-41. [Crossref] [PubMed]
- Dhouha B, Ahlem L, Sana BS, et al. Unexpected cause for duodenal obstruction: Brunner’s gland hyperplasia. Pathologica 2017;109:414-7. [PubMed]
- Asayama Y, Fang W, Stolpen A, et al. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emerg Radiol 2012;19:121-5. [Crossref] [PubMed]
- Manfredi R, Costamagna G, Brizi MG, et al. Pancreas divisum and “santorinicele”: diagnosis with dynamic MR cholangiopancreatography with secretin stimulation. Radiology 2000;217:403-8. [Crossref] [PubMed]
- Hellerhoff KJ, Helmberger H 3rd, Rösch T, et al. Dynamic MR pancreatography after secretin administration: image quality and diagnostic accuracy. AJR Am J Roentgenol 2002;179:121-9. [Crossref] [PubMed]
- Iemoto T, Shiomi H, Masuda A, et al. A case of groove pancreatitis associated with duodenal ulcer. Nihon Shokakibyo Gakkai Zasshi 2013;110:88-94. [PubMed]
- Fujita N, Shirai Y, Tsukada K, et al. Groove pancreatitis with recurrent duodenal obstruction. Report of a case successfully treated with pylorus-preserving pancreaticoduodenectomy. Int J Pancreatol 1997;21:185-8. [PubMed]
- Tezuka K, Makino T, Hirai I, et al. Groove pancreatitis. Dig Surg 2010;27:149-52. [Crossref] [PubMed]
- Nankoe SR, Wilcox R, Roggin KK. Paraduodenal pancreatitis (groove pancreatitis) mimicking pancreatic adenocarcinoma. Clin Gastroenterol Hepatol 2012;10:A31-2. [Crossref] [PubMed]
- Latham J, Sanjay P, Watt DG. Groove pancreatitis: A case series and review of the literature Scott Med J 2013;58:e28-31. [Crossref] [PubMed]
- Casetti L, Bassi C, Salvia R, et al. “Paraduodenal” pancreatitis: Results of surgery on 58 consecutive patients from a single institution. World J Surg 2009;33:2664-9. [Crossref] [PubMed]
- Shudo R, Yazaki Y, Sakurai S, et al. Groove pancreatitis: Report of a case and review of the clinical and radiologic features of groove pancreatitis reported in Japan. Intern Med 2002;41:537-42. [Crossref] [PubMed]
- Ferreira A, Remalho M, Herédia V, et al. Groove pancreatitis: A case report and review of the literature. J Radiol Case Rep 2010;4:9-17. [Crossref] [PubMed]
- Chatelain D, Vibert E, Yzet T, et al. Groove pancreatitis and pancreatic heterotopia in the minor duodenal papilla. Pancreas 2005;30:e92-5. [Crossref] [PubMed]
- Glaser M, Roskar Z, Skalicky M, et al. Cystic dystrophy of the duodenal wall in a heterotopic pancreas. Wien Klin Wochenschr 2002;114:1013-6. [PubMed]
- Itoh S, Yamakawa K, Shimamoto K, et al. CT findings in groove pancreatitis: correlation with histopathological findings. J Comput Assist Tomogr 1994;18:911-5. [Crossref] [PubMed]
- Kwak SW, Kim S, Lee JW. Evaluation of unusual causes of pancreatitis: role of cross-sectional imaging. Eur J Radiol 2009;71:296-312. [Crossref] [PubMed]
- Rahman SH, Verbeke CS, Gomez D, et al. Pancreatico-duodenectomy for complicated groove pancreatitis. HPB (Oxford) 2007;9:229-34. [Crossref] [PubMed]
- Vullierme MP, Vilgrain V, Flejou JF, et al. Cystic dystrophy of the duodenal wall in the heterotopic pancreas: radiopathological correlations J Comput Assist Tomogr 2000;24:635-43. [Crossref] [PubMed]
- Ray S, Ghatak S, Misra D, et al. Groove Pancreatitis: Report of Three Cases with Brief Review of Literature. Indian J Surg 2017;79:344-8. [Crossref] [PubMed]
- Coakley FV, Hanley-Knutson K, Mongan J, et al. Pancreatic Imaging mimics: Part 1, Imaging mimics of pancreatic adenocarcinoma. AJR Am J Roentgenol 2012;199:301-8. [Crossref] [PubMed]
- Hernández-Jover D, Pernas JC, González-Ceballos S, et al. Pancreatoduodenal junction: Review of anatomy and pathologic conditions. J Gastrointest Surg 2011;15:1269-81. [Crossref] [PubMed]
- Desai GS, Phadke A, Kulkarni D. Cystic Dystrophy of the Duodenum Due to Heterotopic Pancreas - A Case Report and Review of Literature. J Clin Diagn Res 2016;10:PD11-3. [PubMed]
- Ishigami K, Taiima T, Nishie A, et al. Differential diagnosis of groove pancreatic carcinomas vs. groove pancreatitis: usefulness of the portal venous phase. Eur J Radiol 2010;74:e95-100. [Crossref] [PubMed]
- Boraschi P, Donati F, Cervelli R, et al. Secretin-stimulated MR cholangiopancreatography: spectrum of findings in pancreatic diseases. Insights Imaging 2016;7:819-29. [Crossref] [PubMed]
- El-Nekidy AE-AM, Ibrahim ME, Abdelgawad MS, et al. Groove pancreatitis: Imaging features and management. Egypt J Radiol Nucl Med 2016;47:1175-84. [Crossref]
- Kalb B, Martin DR, Sarmiento JM, et al. Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology 2013;269:475-81. [Crossref] [PubMed]
- Iglesias García J, Larino Noia J, Dominguez-Munoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig 2009;101:631-8. [Crossref] [PubMed]
- Chute DJ, Stelow EB. Fine-needle aspiration features of paraduodenal pancreatitis (groove pancreatitis): a report of three cases. Diagn Cytopathol 2012;40:1116-21. [Crossref] [PubMed]
- Brosens LA, Leguit RJ, Vleggaar FP, et al. EUS-guided FNA cytology diagnosis of paraduodenal pancreatitis (groove pancreatitis) with numerous giant cells: conservative management allowed by cytological and radiological correlation. Cytopathology 2015;26:122-5. [Crossref] [PubMed]
- Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging 2008;33:342-8. [Crossref] [PubMed]
- Laugier R, Grandval P. Does paraduodenal pancreatitis systematically need surgery? Endoscopy 2014;46:588-90. [Crossref] [PubMed]
- Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis). Radiol Clin North Am 2012;50:447-66. [Crossref] [PubMed]
- Shanbhogue AK, Fasih N, Surabhi VR, et al. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics 2009;29:1003-26. [Crossref] [PubMed]
- Gabata T, Kadoya M, Terayama N, et al. Groove pancreatic carcinomas: radiological and pathological findings. Eur Radiol 2003;13:1679-84. [Crossref] [PubMed]
- Semelka RC, Custodio CM, Cem Balci N, et al. Neuroendocrine tumors of the pancreas: spectrum of appearances on MRI. J Magn Reson Imaging 2000;11:141-8. [Crossref] [PubMed]
- Yu J, Fulcher AS, Turner MA, et al. Normal anatomy and disease processes of the pancreatoduodenal groove: imaging features. AJR Am J Roentgenol 2004;183:839-46. [Crossref] [PubMed]


