Retroperitoneal adrenalectomy—learning curve, practical tips and tricks, what limits its wider uptake
Introduction
Following the introduction of minimally invasive surgery in the early 1980s, it took about ten years to see this innovative approach to be applied in the endocrine surgery, as the first description of laparoscopic adrenalectomy was published in 1992 by Higashihara in Japan (1) and by Gagner in Canada (2). During the same year a minimally invasive retroperitoneal approach was described by Gaur for urologic procedures including uretero-lithotomy, renoscopy, renal biopsy, para-aortic lymph node biopsy and ligation of the internal spermatic vein (3). In 1993 Brunt et al. developed a technique for endoscopic adrenalectomy in a domestic swine model using insufflation of the retroperitoneal space with CO2 and retroperitoneoscopy (4). The results of this study suggested that the posterior route could have been potentially suitable to the treatment of adrenal lesions. In 1994 retroperitoneal adrenalectomy in humans has been described in Japan, New Zeeland and Sweden (5-7).
The obvious advantages of the minimally invasive approach, either laparoscopic or retroperitoneoscopic, in terms of postoperative pain, reduced morbidity and shorter hospital stay have made this operation the procedure of choice for small adrenal tumours (8,9).
As in the era of open surgery, there is still an on-going discussion whether the anterior or posterior approach represents the best route to the adrenal gland. Laparoscopic transperitoneal adrenalectomy has been demonstrated to be a safe and standardized procedure with a short learning curve and low morbidity rate. Nevertheless, many studies indicate that posterior retroperitoneoscopic adrenalectomy is superior to laparoscopic one regarding operation time, pain score, blood loss, complications rate and return to normal activity (10). A recent meta-analysis suggests that retroperitoneoscopic adrenalectomy has equivalent outcome to laparoscopic surgery but may be associated with a shorter hospital stay (11). According to the position of the patient (lateral decubitus versus prone position) two different technique of retroperitoneal adrenalectomy have been described in the second half of the ‘90s (12). The results of retroperitoneoscopic adrenalectomy in lateral decubitus were reported to be very promising in a large series published by Bonjer and co-workers (13). Nevertheless, the operation failed to obtain wide acceptance. On contrary, the retroperitoneoscopic adrenalectomy in prone position has been developed by Walz into a standardised technique (14), which has gained a recognised place worldwide over the last 15 years.
The technique of retroperitoneoscopic adrenalectomy
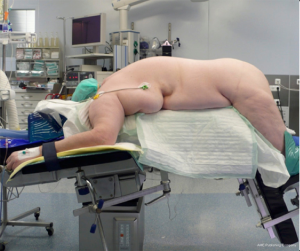
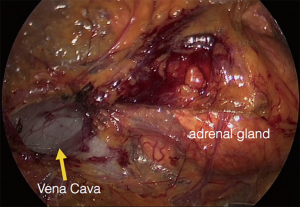
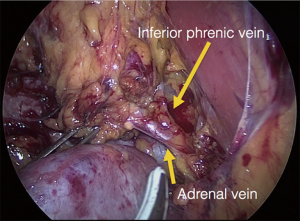
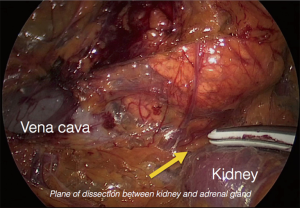
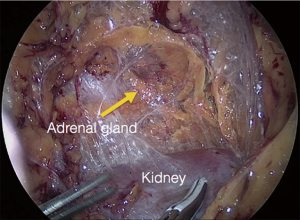
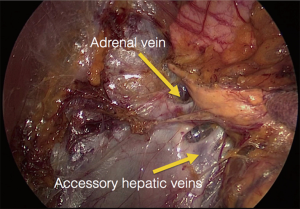
The procedure is performed with the patient in prone position lying on a rectangular support empty in the middle that allows the abdominal wall to hang ventrally through with a 90° angle between the body and the legs (Figure 1). A single shot antibiotic is given before induction of the anaesthesia. A 1.5 cm skin incision is performed at the level of the 12th rib and the retroperitoneal space is reached by blunt and sharp dissection with scissors. A finger is than inserted into the space and the tip of the eleventh rip is localized. A 5 mm trocar is inserted under finger control just below the tip of the 11th rip. At the beginning of the experience the third trocar was placed, at the same level of the first one, under finger guidance without visual control at half distance between the spine and the first incision (14). Actually the technique has been modified and CO2 insufflation at a pressure of 20 mmHg is started before the insertion of the third trocar. A blunt trocar with an inflatable balloon and an adjustable sleeve (Medtronic, Minneapolis, USA) is therefore introduced into the initial incision site and blocked. The working space is created by blunt dissection with the camera looking at the left diaphragmatic crus and consequently the 10 mm trocar is inserted under the view of the endoscope. This permits the visual identification of the subcostal nerve and avoids therefore its lesion. The position of the trocars is showed in Figure 2. The originally described method of balloon dilatation was stopped already at an early stage. Retroperitoneoscopy is performed by a 10 mm 30° endoscope, although in selected cases a 5 mm scope can be used. The 5 mm 30° endoscope is also adopted for the single access operation (SARA) described in 2009 (15). The Ligasure Maryland with curved tip (Medtronic, Minneapolis, USA) is actually the preferred instrument for dissection and vessels sealing. The first step of the procedure is the visualization and mobilization of the upper pole of the kidney (Figures 3,4). According to the different position of the adrenal gland between right and left side, extensive mobilization of the kidney is generally required on the left side while the adrenal is mostly in a suprarenal position on the right side. The dissection of the adrenal gland should start at the lower pole and achieved from lateral to medial. By this approach the vena cava is visualized on the right side and the adrenal vein on the left side (Figures 5,6). On the right side the retrocaval arteries are divided and the dissection is continued cranially to identify the adrenal vein (Figure 7). The manipulation of the gland is always performed carefully using blunt palpation probes to prevent injury to the capsule. The main adrenal vein is isolated and divided after coagulation without application of clips. The dissection is than completed cranially and ventrally taking care not to damage the Toldt’s membrane and the peritoneal layer. The adrenal gland is extracted through the middle incision with a retrieval bag system (Endocatch, Medtronic, Minneapolis, USA). Depending on tumor size and on the underlying pathology morcellation of the tumor can be required. After optional drain insertion, skin and fascia are closed with reabsorbable sutures.
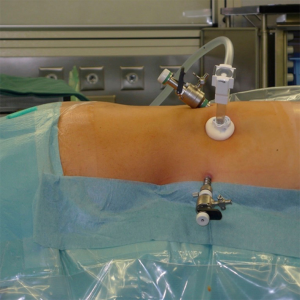
Bilateral tumors can be removed without repositioning of the patient necessary resulting in a significant reduction of the operating time if two teams can operate simultaneously.
During subtotal adrenalectomy the adrenal gland is dissected according to the position of the tumor preserving the adrenal arteries feeding the normal tissue. Preservation of the main vein is not necessary (16). To identify the margin of the neoplasia, it may be necessary to dissect or resect parts of the surrounding fatty tissue, which is normally resected “en-bloc” with the gland in case of total adrenalectomy. Retroperitoneoscopic ultrasonography by a 10 mm flexible 10 MHz probe can be applied individually for identification of the neoplasms. Division of the adrenal tissue is performed by bipolar coagulation. The insertion of drainage is generally not required.
Learning curve, practical tip and tricks
The complications rate of adrenalectomy ranges from 0% to 15% for unilateral operation and rises up to 23% for bilateral surgery. No significant differences are found between laparoscopic and retroperitoneoscopic operations. Nevertheless, splenic injuries and intra-abdominal abscesses are reported only after laparoscopic procedures, while relaxation and/or hypoesthesia of the abdominal wall are typical for posterior retroperitoneoscopic surgery (17). Switching from the anterior to the posterior view could be related with several troubles connected to the different anatomical view, the small working space and the limited degree of freedom of the laparoscopic instruments inserted below the ribs. Laparoscopic adrenalectomy has the advantage of a familiar anatomical view coupled to a wide working space.
The learning curve for laparoscopic adrenalectomy is reported to be of approximately 30 cases, if performed by an experienced laparoscopic surgeon (18). Barczynski and co-workers demonstrated that the introduction of the retroperitoneoscopic adrenalectomy was not related to an increased complications rate or longer operating time when supervised by an experienced team (19). The mean operative time could be reduced from 117 minutes reported during the learning period in Essen to 83 minutes for the initial phase in Krakow. The authors described one conversion and one early reoperation due to bleeding compared to seven conversions in the initial experience by Walz in Essen.
These results have been confirmed in a recent multicentre study undertaken at four endocrine centers. The analysis of the learning curve cumulative sum was used to evaluate the learning curve for the posterior adrenalectomy (20). The median overall duration of operation was 89 (range, 29–265) min. The surgical teams reached competency after 24, 29, 40 and 42 procedures, as demonstrated by crossing the established threshold. Male sex and high BMI were proven to correlate significantly with the duration of surgery.
Increasing experience and several improvements that have modified the originally described operation allowed us to reduce the median operating time to 45 minutes over 2,310 procedures performed between July 1994 and September 2018 and to reduce the complications rate to less than 1%.
Following some practical tips are summarized:
- The position of the patient with a 90° angle between the spine and the legs was adopted from the beginning of the experience and it is of crucial importance to obtain the optimal distance between the rib and the hip bone.
- A correct position of the trocar is necessary to avoid conflict between the instruments during surgery. According to the anatomy of the patient, if the 11th rip is long the trocar could be inserted cranially to the rip to allow a better degree of freedom for the instruments. The insertion of the medial trocar should be performed with a flat angle under optical view to avoid lesion of the subcostal nerve and accidentally insertion of the trocar into the chest.
- CO2 insufflation pressure can be increased up to 30 mmHg and modulated according to the anatomy of the patient and the diameter of the tumor to increase the space as required without side effects for the patient. Increase of the pressure can be also used in case of bleeding to obtain temporary haemostasis due to the compression of the small vessels.
- The dissection should be always started at the upper pole of the kidney. A wide mobilisation is necessary on the left side prior to approach the adrenal gland. The lower pole of the adrenal gland is generally automatically visualized and dissected leaving the adrenal attached to the lateral and upper adhesions. Division of the retrocaval arteries on the right side can facilitate the exposition of the vena cava and of the retrocaval portion of the lower pole of the right adrenal gland. The dissection should than be conducted from 3 to 9 hours clockwise on the right side and from 9 to 3 hours counterclockwise on the left side.
- During the learning period it can be reasonably recommended not to approach tumors larger than 6 cm, as the tumor diameter is the most important prognostic factor for malignancy. Radiologic features suggestive of malignancy are generally not specific enough to discourage a minimally invasive approach for tumors <6 cm.
- A BMI (body mass index) of more than 35 represents a relative contraindication during the learning phase. The dense retroperitoneal fat, especially in male patients, is generally attached very strongly to the capsule of the kidney and can make the dissection very difficult.
The ability to visit an institution with expertise in the technique can be helpful to successful adoption of the procedure in the own institution (20,21).
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Higashihara E, Tanaka Y, Horie S, et al. A case report of laparoscopic adrenalectomy. Nihon Hinyokika Gakkai Zasshi 1992;83:1130-3. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Gaur DD. Laparoscopic operative retroperitoneoscopy: use of a new device. J Urol 1992;148:1137-9. [Crossref] [PubMed]
- Brunt LM, Molmenti EP, Kerbl K, et al. Retroperitoneal endoscopic adrenalectomy: an experimental study. Surg Laparosc Endosc 1993;3:300-6. [PubMed]
- Uchida M, Imaide Y, Yoneda K, et al. Endoscopic adrenalectomy by retroperitoneal approach for primary aldosteronism. Hinyokika Kiyo 1994;40:43-6. [PubMed]
- Whittle DE, Schroeder D, Purchas SH, et al. Laparoscopic retroperitoneal left adrenalectomy in a patient with Cushing's syndrome. Aust N Z J Surg 1994;64:375-6. [Crossref] [PubMed]
- Johansson K, Anderberg B, Asberg B. Endoscopic retroperitoneal adrenalectomy. A technique useful for surgery of minor tumors. Lakartidningen 1994;91:3278, 3281.
- Brunt LM, Doherty GM, Norton JA, et al. Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms. J Am Coll Surg 1996;183:1-10. [PubMed]
- Walz MK, Giebler RM. Endoscopic, retroperitoneal adrenalectomy. Chirurg 1997;68:650. [PubMed]
- Walz MK. Minimally invasive adrenal gland surgery. Transperitoneal or retroperitoneal approach? Chirurg 2012;83:536-45. [Crossref] [PubMed]
- Constantinides VA, Christakis I, Touska P, et al. Systematic review and meta-analysis of retroperitoneoscopic versus laparoscopic adrenalectomy. Br J Surg 2012;99:1639-48. [Crossref] [PubMed]
- Walz MK, Peitgen K, Hoermann R, et al. Posterior retroperitoneoscopy as a new minimally invasive approach for adrenalectomy: results of 30 adrenalectomies in 27 patients. World J Surg 1996;20:769-74. [Crossref] [PubMed]
- Bonjer HJ, Sorm V, Berends FJ, et al. Endoscopic retroperitoneal adrenalectomy: lessons learned from 111 consecutive cases. Ann Surg 2000;232:796-803. [Crossref] [PubMed]
- Walz MK, Peitgen K, Walz MV, et al. Posterior retroperitoneoscopic adrenalectomy: lessons learned within five years. World J Surg 2001;25:728-34. [Crossref] [PubMed]
- Walz MK, Alesina PF. Single access retroperitoneoscopic adrenalectomy (SARA)--one step beyond in endocrine surgery. Langenbecks Arch Surg 2009;394:447-50. [Crossref] [PubMed]
- Brauckhoff M, Gimm O, Thanh PN, et al. Critical size of residual adrenal tissue and recovery from impaired early postoperative adrenocortical function after subtotal bilateral adrenalectomy. Surgery 2003;134:1020-7; discussion 1027-8. [Crossref] [PubMed]
- Alesina PF. Complications of minimally invasive adrenalectomy. Chirurg 2015;86:29-32. [Crossref] [PubMed]
- Goitein D, Mintz Y, Gross D, et al. Laparoscopic adrenalectomy: ascending the learning curve. Surg Endosc 2004;18:771-3. [Crossref] [PubMed]
- Barczynski M, Konturek A, Golkowski F, et al. Posterior retroperitoneoscopic adrenalectomy: a comparison between the initial experience in the invention phase and introductory phase of the new surgical technique. World J Surg 2007;31:65-71. [Crossref] [PubMed]
- Vrielink OM, Engelsman AF, Hemmer PHJ, et al. Multicentre study evaluating the surgical learning curve for posterior retroperitoneoscopic adrenalectomy. Br J Surg 2018;105:544-51. [Crossref] [PubMed]
- Perrier ND, Kennamer DL, Bao R, et al. Posterior retroperitoneoscopic adrenalectomy: preferred technique for removal of benign tumors and isolated metastases. Ann Surg 2008;248:666-74. [PubMed]