
Gd-EOB-DTP-enhanced MRC in the preoperative percutaneous management of intra and extrahepatic biliary leakages: does it matter?
Introduction
Postoperative bile leakage is a common complication following upper abdominal surgery. The incidence rates are strictly linked with the type of performed surgery, varying between 0.9% and 9.0% (1), with rates of mortality reported between 8.7% and 39.0% and morbidity ranging between 22% and 44% (2). The endoscopic approach based on endoscopic retrograde cholangiopancreatography (ERCP) is widely considered the primary choice to solve bile leaks, being in many cases the percutaneous transhepatic biliary drainage (PTBD) a useful secondary tool after ERCP failed attempts or when an ERCP approach is not feasible (3). However, try to obtain a safe biliary access in patients with nondilated bile ducts, such as in case of bile leakages, is technically more complex of dilated ones, being necessary many punctures of liver parenchyma with a higher risk of severe bleedings (4). Many studies have shown that PTBD can be achieved in patients without dilated bile ducts with success rates not far from those with dilated ones (90.5%) (5). The initial diagnosis of a bile leakage in many cases follows the presence of bile in an abdominal drain or the presence of fever with abdominal pain or sepsis with or without an evident peritonitis confirmed by an ERCP or a PTC (6,7). A precise localization of bile leaks plays a pivotal rule to choose the best management, being available many imaging modalities ranging from first line ones, like US, to more expensive, like CT or MRI (8). All these techniques, chosen in the right clinical setting, are able to give useful findings highly suggestive for a bile leak, being the contrast-enhanced Magnetic Resonance Cholangiography (CE-MRC) the best one to obtain clear images of an active bile leakage or of a cysto-biliary communication. The aim is to identify the source of a contrast media (CM) extravasation and the precise site of the leakage with associated bile duct injury (9). When a clear site of active bile leakage is not well shown, invasive techniques such as PTC or ERCP are required to show an active CM extravasation from the bile ducts.
Our aim is to describe the role of CE-enhanced MRC with T2w-MRC or alone, in the setting of preoperative percutaneous management of intra and extrahepatic biliary leakages, resuming available techniques, tips, tricks and typical findings.
Current algorithm in the detection of biliary leakages
The current algorithm to identify a suspected bile leak is to perform abdominal US followed by CT and MRC when a fluid collection is detected. The common finding is the presence of a biloma, characterized on MR by a fluid collection sometimes with high T1 and low T2 signal due to layering of concentrated extravasated bile (10). T2w-MRC alone is able to depict a bile leak in the range of 55% and 65% (11), whilst CE-MRC with liver specific contrast agent can show the source of biliary leak separating fluid collections by biliary ones. When a bile leak is suspected, a positive diagnosis is made if the contrast agent is visible at the same time on late hepatocyte-specific phases in the biliary tree and outside the bile ducts. However, must be considered that the most of the studies available in literature were performed using Mn-DPDP, a liver-specific CM no more available in Europe and US markets, respectively from 2012 and 2003. To date two liver-specific agents are still available in the US and Europe: Gd-EOB-DTPA and Gd-BOPTA, even if according to European Medicine Agency recommendations released in 2017, the intravenous linear agent Gd-BOPTA should be used in body imaging only for liver scans, in those situations where its use is strictly advocated, shifting the users “de facto” to perform scans with safer and easily available gadoxetic acid (Gd-EOB-DTPA) remaining Gd-BOPTA as second choice, despite lower costs.
When considering the integration of gadoxetic acid scans in a protocol based on T2-weighted (T2w) MRC it should be noted that its use is admitted for the detection and characterization of focal liver lesions being the depiction of bile leaks an “off label” use. In this setting Kul et al. showed that the sensitivity, specificity, and accuracy were definitely higher when compared with T2W-MRC alone and equal to 92.9%, 90.5%, and 100%. Alegre Castellanos et al. found that Gd-EOB-DTPA boosted bile leaks detection to 100% of cases if performed 20 minutes from baseline scans, with a highly reliable and advantageous technique over invasive strategies (12). Due to the high spatial resolution available with Gd-EOB-DTPA-enhanced MRC, it can be considered useful to determine the type ductal injury, the presence of accessory bile ducts or subvesicular that can be a common source of bile leakage (13). Moreover, following European Medicine Agency recommendations concerning Gd-BOPTA and the lack of Mn-DPDP on EU and US markets, in this review will be considered only studies performed with gadoxetic acid, being easily to be retrieved in clinical routine due to the wider availability on the market of the latter compound.
To perform a Gd-EOB-DTPA-enhanced MRC useful to plan a prompt leakage management, avoiding more invasive or unnecessary tests, is mandatory solve some technical issues regarding its pharmacokinetic properties conditioning the choice of an adequate scan time to improve biliary tree/background visualization ratio and the set of an optimal flip angle to optimize SNR ratio.
Gadoxetic acid pharmacokinetic properties and their impact biliary imaging
Gadolinium hepatocyte-specific CM are cleared by glomerular and biliary excretion: in particular for the gadoxetic acid the biliary amount is almost 50%, which is more than other liver-specific available compounds (14). The biliary tree is well shown in the late phase with T1-weighted gradient echo scans that demonstrate smaller peripheral branches in a phase named “excretory phase”. The recommended dose for gadoxetic acid is one quarter of a typical extracellular CM, corresponding to 0.025 mmol/kg, thus requiring more care to obtain an optimal arterial phase (AP) reducing its flow rate or through its dilution with saline solution. However, the AP protocol is outside of the aims of this review, so our attention will be focused on factors that impact on the quality of images obtained during “excretory/liver specific phase”. Many authors recommend to obtain scans from 20 minutes from injection (9), because the choice of the optimal time window for “liver specific phase” scans and the presence or not of a clear bile leakage, clearly impact on the execution time of a Gd-EOB-DTPA-enhanced MRC. Cieszanowski et al. in their series performed delayed phase (DP) scans at different times after Gd-EOB-DTPA injection (at 20–25, 90 and 60–180 minutes). They showed the importance of 60–180 minutes DP, calculating the sensitivity in depicting bile leaks for each DP scans, showing a statistically significant difference between the sensitivity of 20–25 minutes alone, the combined 20–25 and 60–90 minutes or of the complete examination carried out by obtaining scans at 20–25, 60–90 and 150–180 minutes (42.9%, 92.9% and 96.4% respectively) (15).
When there is a clinical significant suspicion of bile leakage, but liver specific phase can’t demonstrate it, Kul et al. suggested to perform supplementary DP scan from 30 minutes after the Gd-EOB-DTPA injection as long as a biliary leakage is found or up to 24 hours, on the basis of the radiologist’s opinion. In their series, times of additional scans in which leaks were showed ranged from 60–90, 120–150, 210–240 to 390 minutes (the most delayed phase). In very suspicion cases, they indicate that DP scans should be done even if the patient has normal total bilirubin levels and liver function tests. In fact three patients with bile leaks evidence during the DP, had normal laboratory results and no bile ducts enlargement. On the other hand patients with biliary leakage detected in DP scans had generally higher levels of total bilirubin (16). Although more studies are needed, some hypothesis are been made to explain the necessary use of DP images: a non-specific hepatocyte dysfunction could cause high alkaline phosphatase (ALP) and alanine aminotransferase (ALT) values or high bilirubin values may lead a reduced hepatocyte-uptake of Gd-EOB-DTPA, with a delayed visualization of the biliary ducts and potential bile leaks (17). Moreover, in case of bile duct obstruction, the up-regulation of a multidrug resistance protein, (MRP3) could reduce the excretion of Gd-EOB-DTPA in the blood mainstream, delaying or preventing the visualization of bile ducts and bile leakage (18). In conclusion, even if hepatic enzymes and bilirubin values are regular without enlarged biliary tree, additional delayed phase images (>30 minutes) should be performed if the biliary leakage suspicion is significant.
Common sites of leakage after surgery: what interventional radiologists should look for
After cholecystectomy biliary leakage can occur as an important complication (19-21) with an incidence ranging from 0.2% to 7% for laparoscopic cholecystectomy and from 0.1% to 0.5% for open cholecystectomy (19-24). Bile leak becomes manifest with one or more of these clinical symptoms: abdominal pain (the common one), fever, bilious drain, anorexia, malaise, nausea, vomiting and jaundice with or without the increase in bilirubin, transaminases and in leukocytes (12,16,25). After cholecystectomy, 7 days is the medium demonstration time (ranging, usually is 1–15 days, but also 30 days after the surgery) (12,16,24), carrying an increase in morbidity and mortality, and leading not only to a diminution of long-term survival but also a worse quality of life (19-21). The use CE-MRCP has been widely used over the time even if most of the studies has been based on Mangafodipir-trisodium and just some limited number of papers that utilize Gd-EOB-DTPA have been published (12,24,26,27).
Gd-EOB-DTPA gives the possibility to perform at the same time a dynamic contrast-enhanced MR study coupled with delayed liver specific scans, able to depict a wide spectrum of complications related to parenchyma, vascular structures or bile ducts during the same examination (24,28). A retrospective review performed by Ratcliffe et al. showed a 100% sensitivity and 98% specificity in depicting bile leaks after cholecystectomy by performing MRC with iv Gd-EOB-DTPA. The only false positive was due by Gd-EOB-DTPA pooling in the cystic duct stump misdiagnosed as a bile leak (24). In literature there are some case reports which emphasize the role of CE-MRI in achieving the correct diagnosis of post-cholecystectomy bile leak (29-32). Other authors included cholecystectomy patients in their series recommending combined use of T2w-MRC and Gadoxetic Acid administration to increase the accuracy in depicting the sites of bile leaks (11,12,16). For what concerns common extravasation sites following hepato-biliary surgery, the extravasated bile may be contained in a well-circumscribed collection (biloma) or may leak freely into the peritoneal space. The most common location for biloma is in the sub hepatic area, however, it can be in the intrahepatic or, rarely, in the retroperitoneal space (26,33,34). The pooling of contrast media inside bilomas can slightly increase their signal intensity or create areas of strong hyperintensity on T1-weighted gradient echo scans as results of free extravasation outside liver parenchyma (26). When patients are in supine position, being the CM less dense than bile, it tends to accumulate in the anterior part of the fluid collections, following the same behavior seen inside gallbladder in healthy patients (26,33). Even if bilomas are found near to the site of a bile duct injury, sometimes they could be located far from primary damaged site. Serial imaging when contrast starts appearing in the biliary tree helps to accurately localize the site of the leak before the contrast spreads away (24). Kul et al. reported one case of cholecystectomy in which bile leak was detected after a delay of 390 minutes after Gd-EOB-DTPA administration (16,24). However, free or collected fluid located near liver or adjacent to a damaged bile duct or on the abdominal right side could be strongly indicative of a bile leak. In this setting common associated injuries are partial or complete bile duct transactions being the lack of a bile duct segment on MRCP scans highly suspicious for an “injury excision” (16,25,29).
There are several classifications after the first from Bismuth used by radiologists to relate the leakage sites with CE-MRC (27) and damages after cholecystectomy (Strasberg classification, Mattox classification, Hannover classification, Stewart-Way classification and McMahon classification) (35,36). Bismuth classification is based on the distance of biliary strictures from the biliary confluence and does not include the entire spectrum of bile duct injuries. Instead, the Strasberg classification permits the differentiation between bile leakage from a small bile duct and serious injuries performed during laparoscopic cholecystectomy (35,36). However the Strasberg classification does not describe additional vascular involvement (29,36).
Most common causes of bile leaks secondary to laparoscopic cholecystectomy include (25): slippage of the cystic duct ligature; leak from an accessory or anomalous bile duct due to incidental injury related to misidentification of biliary anatomy; leaks from the gallbladder bed. The most common site for leak is the cystic duct stump and usually a small collection develop adjacent to the cystic duct remnant or near the cholecystectomy clips. The volume of extravasated contrast usually increases in the late sequences MR images during the liver specific hepatobiliary phase (34,37).
Frequently sites of origin can be subvesical bile ducts, common bile duct (CBD), common hepatic duct, liver bed or intrahepatic ducts (Figure 1). In particular “subvesical bile ducts” (frequently termed incorrectly “ducts of Luschka”), should include “any bile duct traversing in close contact with the gallbladder fossa”, encompassing different types of bile ducts see (Figure 2) (38). The variability in anatomic location of subvesical bile ducts and their difficult visualization on preoperative imaging and during laparoscopy, puts them at risk during hepato-biliary surgery (38,39), especially when the presence of an anatomic abnormality (e.g., an intrahepatic position of the gallbladder, or an adherent gallbladder due to chronic cholecystitis) makes surgical access difficult and when the aberrant subvesicular bile ducts are injured and drained directly to the surgical bed (type 3 or 4) (Figure 1) (25,27,40).
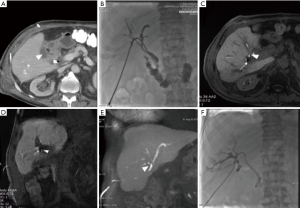
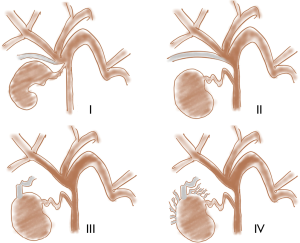
CE-MRC permits to depict leaks that do not communicate with distal biliary ductal system and therefore would not likely be detected with ERCP, because the latter misses those arising from aberrant bile ducts that are disconnected from the main biliary tree (24,26,41-43) (Figure 1).
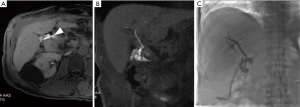
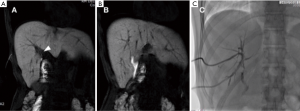
Biliary leakages could be observed after many other surgical procedures, including resection of bile duct tumors, repair of biliary tree injuries, liver transplantation, palliative surgical approaches for unresectable obstructive tumors, pancreaticoduodenectomy for benign and malignant neoplasms (Figure 3) and many other surgical procedures for chronic pancreatitis and choledocholithiasis in which hepaticojejunostomy are performed (Figure 4). The latter is usually performed with anastomoses between a normal bile duct segment proximal to the site of disease and a Roux-en-Y loop of the jejunum (44), without cystic duct remnant, although a very small remnant may be left in place. Biliary leaks after hepaticojejunal anastomosis are rare events, but with potential greater morbidity (44), occurring in 0.4% to 8% of the patients, depending on the type of procedure (7) with a median onset around day 4 (ranging between 2–13 days) after the index operation (45). De Castro et al., showed that biliary leaks occurred in 24 of 1,033 patients (2.3%) after hepaticojejunostomy with an incidence varying from 11% after proximal bile duct resection to 1% after a palliative bypass procedure for malignant disease (7). In a series of Antolovic et al., among 519 patients analyzed, bile leak rates was of 0 to 5% for patients undergoing pancreaticoduodenectomy or pancreatectomy and for patients undergoing hepaticojejunostomy for repair of bile duct injury, while biliary leaks, in the context of liver transplantation, occurred in 1–25% of patients (based on type of biliary reconstruction, hepaticojejunostomy or choledocho-choledochostomy) (45). When dealing with bile leak potentially arising from bilioenteric anastomosis great advantages were shown introducing contrast-enhancement MR Cholangiography (46). Wellner et al. showed different sites where search the origin of a bile leak because in the setting of bilioenteric anastomosis the source of biliary secretions may occur also from other sites even if injuries of the biliary tree outside of the anastomosis region are rare. Rather rare are combined leaks of bile and pancreatic juice after procedural lacerations of the small bowel after surgery. After pancreatoduodenectomy, biliary leakage may also be derived from an insufficient pancreatojejunostomy, especially when a single jejunal loop is used for bilioenteric and pancreatoenteric anastomosis and the distance between the pancreatic and the biliary anastomosis is short. Therefore, the localization of the bile leak is mandatory before considering reoperation or a percutaneous drainage (44).
CE-MRC: what keep in mind
The parameters of the hepatobiliary phase carried in the suspicion of a biliary leak could be modified in order to optimize biliary tree visualization. For instance, by increasing the flip angle the contrast between normal liver parenchyma with gadoxetic acid uptake and excretion is effectively increased. These effects are mainly due to the different pharmacokinetic properties of the Gadoxetic Acid involving in the liver, a multidrug resistance-associated proteins complex (MRPs) able to excrete an half of the contrast media by the liver and an equal percentage by the kidneys (47). A key factor to obtain an optimal enhancement is a preserved hepatobiliary function: in this setting the maximum enhancement of the liver could be obtained in 20 minutes after contrast media administration. A strategy to maximize the liver to biliary duct visualization ratio is based on the use of an increased flip angle, strongly influencing the quality of hepatobiliary acquisitions, especially if dealing with liver with a preserved hepatobiliary function. In this setting clear differences were appreciated when measuring the difference in signal obtained from hepatic parenchyma, bile duct, and muscles, on hepatobiliary images scanned with variable flip angles after 20 minutes from gadoxetic acid administration (48). The peak of intensities for biliary ducts were shown using a flip angle included between 35–40°, whilst the higher signal for the “background” (e.g., liver healthy parenchyma) was obtained lowering the flip angle to interval include between 25–30° (49). The importance of the choice of a correct flip angle after gadoxetic acid administration to improve the visualization of the liver parenchyma or the bile ducts in healthy patients was studied with interesting results reporting 25–30° for relative liver signal versus muscle ratio and 45° for relative bile duct signal vs. liver parenchyma ratio (50). Basing on the concept that relatively higher flip angles could improve bile duct visualization, were compared late hepatobiliary scans obtained with different flip angles ranging between 10° to 35°, focusing the study on the evaluation of the biliary ducts. Better results were obtained in those scans performed using a 35° flip angle, which gave the ability to depict also smaller and distal biliary branches (51). The same author tried to evaluate 3D TSE T2-weighted sequences and hepatobiliary T1-weighted scans obtained 20 minutes after Gadoxetic Acid with a flip angle of 35°. The aim was established which technique could be better to evaluate the entire biliary tree: late phase scans were able to give clearer images with overall higher scores, helping in the depiction of biliary variants with a higher level confidence that usually obtained with T2 isotropic scans (24). Similar results were obtained in another study focused to perform a qualitative and quantitative comparison of image quality in depicting differences among first-, second-, and third-order intrahepatic ducts using scans obtained with different flip angles. Best results in terms of clarity of bile ducts visualization, background signal suppression, and overall image quality were significantly higher for flip angle of 25° and 40°, especially if compared with 12° set during scans carried for focal liver lesion detection and characterization. Same results were obtained for quantitative measurement in Signal to Noise Ratios and Contrast to Noise Ratios of the common bile duct that were significantly higher for flip angle of 25° and 40° than for 12° (25). Another interesting factor to be considered, when using hepatobiliary T1-weighted acquisitions obtained 20 minutes after gadoxetic acid administration concerns the choice of “breath hold” scan or a high-resolution navigated one based on optimized T1-weighted pulse. An interesting paper explored the difference between two different acquisitions using a high spatial resolution T1-weighted sequence of 1.2×1.4×1.8 mm3 interpolated to 0.7×0.7×0.9 mm3 with navigator-gating enabled acquired in approximately 5 minutes of free-breathing and multiple breath-held acquisitions performed at flip angles varying between 15° and 45° to optimize T1-weighting. The aim was to assess the image quality and biliary excretion and results showed that optimal hepatobiliary imaging occurred at 15–25 minutes using a 40° flip angle resulting in an excellent image quality and visualization of biliary excretion.
Special considerations: what to do in cirrhotic liver
The main theory concerning regarding Gadoxetic Acid is that liver specific uptake is based on the organic anion-transporting polypeptide functions of OATP8 (52). In the setting of impaired liver function it is reduced with an overall loss of effective uptake corresponding to a reduced enhancement in the liver specific phase, when liver to muscle ratio is considered. This phenomenon is more prominent when comparing patients with different Child-Pugh class (e.g., A and C) even if there is a lack of data concerning a quantitative difference in the enhancement of cirrhotic with Child-Pugh class A or B disease (53). Some authors suggested to compensate the lack of enhancement by doubling the dose for Child-Pugh class B patients from 0.025 to 0.05 mmol/kg of body weight, prolonging the liver specific phase over conventional 20 minutes after CM injection, a strategy strictly needed, when dealing with bile leakages (54). Must be remembered that in a model for end-stage liver disease with a score ≥11, 76% of examinations were judged not sufficient for anatomical diagnosis of biliary tree after 180 minutes (28). Although in the setting of bile leakages high bilirubin levels is a not frequent condition, their values ≥1.8 mg/dL or 160 µmol/L are described as a factor able to dramatically reduce the image quality (28). The uptake of gadoxetic acid can depend also by the presence of arterial-portal and portal-systemic shunts able to decrease the overall liver blood flow, with consequential GD-EOB-DTPA liver retention (54). Less know causes of a lack in enhancement in liver specific phase, can include severe steatosis, severe fibrosis and iron overload (55,56).
Conclusions
Despite limits of CE-MRC include its high cost and the delay in depicting the bile duct in patients with hepatobiliary dysfunction, the Gd-EOB-DTPA-enhanced MR imaging protocol, when combined with T2-weighted MR cholangiography can definitely improve the visualization of biliary system and bile leaks. This approach must be included in the planning of any radiologic interventional procedure aimed to treat biliary injuries, particularly post-surgical leaks. However, its clinical applications must be widely investigated to clearly define the best technique to better visualize smaller leakages and more studies are needed to standardize the technique to perform Gd-EOB-DTPA-enhanced MR cholangiography in a clinical setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Buanes T, Waage A, Mjåland O, et al. Bile leak after cholecystectomy significance and treatment: results from the National Norwegian Cholecystectomy Registry. Int Surg 1996;81:276-9. [PubMed]
- Yamashita Y, Hamatsu T, Rikimaru T, et al. Bile Leakage After Hepatic Resection. Ann Surg 2001;233:45-50. [Crossref] [PubMed]
- Kim KH, Kim TN. Endoscopic management of bile leakage after cholecystectomy: a single-center experience for 12 years. Clin Endosc 2014;47:248-53. [Crossref] [PubMed]
- Kühn JP, Busemann A, Lerch MM, et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol 2010;195:851-7. [Crossref] [PubMed]
- de Jong EA, Moelker A, Leertouwer T, et al. Percutaneous transhepatic biliary drainage in patients with postsurgical bile leakage and nondilated intrahepatic bile ducts. Dig Surg 2013;30:444-50. [Crossref] [PubMed]
- Hoekstra LT, van Gulik TM, Gouma DJ, et al. Posthepatectomy bile leakage: how to manage. Dig Surg 2012;29:48-53. [Crossref] [PubMed]
- de Castro SM, Kuhlmann KF, Busch OR, et al. Incidence and management of biliary leakage after hepaticojejunostomy. J Gastrointest Surg 2005;9:1163-71; discussion 1171-3. [Crossref] [PubMed]
- Nikpour AM, Knebel RJ, Cheng D. Diagnosis and Management of Postoperative Biliary Leaks. Semin Intervent Radiol 2016;33:307-12. [Crossref] [PubMed]
- Jhaveri K, Cleary S, Audet P, et al. Consensus statements from amultidisciplinary expert panel on the utilization and application of a liver-specific MRI contrast agent (gadoxetic acid). AJR Am J Roentgenol 2015;204:498-509. [Crossref] [PubMed]
- Melamud K, LeBedis CA, Anderson SW, et al. Biliary imaging: multimodality approach to imaging of biliary injuries and their complications. Radiographics 2014;34:613-23. [Crossref] [PubMed]
- Kantarcı M, Pirimoglu B, Karabulut N, et al. Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: comparison with T2-weighted MR cholangiography. Eur Radiol 2013;23:2713-22. [Crossref] [PubMed]
- Alegre Castellanos A, Molina Granados JF, Escribano Fernandez J, et al. Early phase detection of bile leak after hepatobiliary surgery: value of Gd-EOB-DTPA-enhanced MR cholangiography. Abdom Imaging 2012;37:795-802. [Crossref] [PubMed]
- Copelan A, Bahoura L, Tardy F, et al. Etiology, Diagnosis, and Management of Bilomas: A Current Update. Tech Vasc Interv Radiol 2015;18:236-43. [Crossref] [PubMed]
- Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, et al. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology 1992;183:59-64. [Crossref] [PubMed]
- Cieszanowski A, Stadnik A, Lezak A, et al. Detection of active bile leak with Gd-EOB-DTPA enhanced MR cholangiography: comparison of 20-25 min delayed and 60-180 min delayed images. Eur J Radiol 2013;82:2176-82. [Crossref] [PubMed]
- Kul M, Erden A, Atman ED. Diagnostic value of Gd-EOB-DTPA-enhanced MR cholangiography in non-invasive detection of postoperative bile leakage. Br J Radiol 2017;90:20160847. [Crossref] [PubMed]
- Norén B, Dahlström N, Forsgren MF, et al. Visual assessment of biliary excretion of Gd-EOB-DTPA in patients with suspected diffuse liver disease – A biopsy-verified prospective study. Eur J Radiol Open 2015;2:19-25. [Crossref] [PubMed]
- Belinsky MG, Dawson PA, Shchaveleva I, et al. Analysis of the in vivo functions of {Mrp}3. Mol Pharmacol 2005;68:160-8. [PubMed]
- Moore DE, Feurer ID, Holzman MD, et al. Long-term detrimental effect of bile duct injury on health-related quality of life. Arch Surg 2004;139:476-81; discussion 481-2. [Crossref] [PubMed]
- Flum DR, Cheadle A, Prela C, et al. Bile Duct Injury during Cholecystectomy and Survival in Medicare Beneficiaries. JAMA 2003;290:2168-73. [Crossref] [PubMed]
- Boerma D, Rauws EJ, Keulemans YC, et al. Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: A prospective analysis. Ann Surg 2001;234:750-7. [Crossref] [PubMed]
- Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: An audit of 5913 cases. Br J Surg 1996;83:1356-60. [Crossref] [PubMed]
- Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: A national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg 1993;165:9-14. [Crossref] [PubMed]
- Ratcliffe GE, Kirkpatrick ID, Anik Sahni V, et al. Detection and Localization of Bile Duct Leaks After Cholecystectomy Using Gd-EOB-DTPA-Enhanced MR Cholangiography. J Comput Assist Tomogr 2014;38:518-25. [Crossref] [PubMed]
- Hoeffel C, Azizi L, Lewin M, et al. Normal and Pathologic Features of the Postoperative Biliary Tract at 3D MR Cholangiopancreatography and MR Imaging. Radiographics 2006;26:1603-20. [Crossref] [PubMed]
- Aduna M, Larena JA, Martín D, et al. Bile duct leaks after laparoscopic cholecystectomy: Value of contrast-enhanced MRCP. Abdom Imaging 2005;30:480-7. [Crossref] [PubMed]
- Khalid TR, Casillas VJ, Montalvo BM, et al. Using MR cholangiopancreatography to evaluate iatrogenic bile duct injury. AJR Am J Roentgenol 2001;177:1347-52. [Crossref] [PubMed]
- Tschirch FTC, Struwe A, Petrowsky H, et al. Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: Visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol 2008;18:1577-86. [Crossref] [PubMed]
- Mungai F, Berti V, Colagrande S. Bile leak after elective laparoscopic cholecystectomy: Role of MR imaging. J Radiol Case Rep 2013;7:25-32. [Crossref] [PubMed]
- Kalayci C, Aisen A, Canal D, et al. Magnetic resonance cholangiopancreatography documents bile leak site after cholecystectomy in patients with aberrant right hepatic duct were ERCP fails. Gastrointest Endosc 2000;52:277-81. [Crossref] [PubMed]
- Marin D, Bova V, Agnello F, et al. Gadoxetate disodium-enhanced magnetic resonance cholangiography for the noninvasive detection of an active bile duct leak after laparoscopic cholecystectomy. J Comput Assist Tomogr 2010;34:213-6. [Crossref] [PubMed]
- McArthur C, Unnam S, Felsenstein I. Isolated blunt traumatic gallbladder perforation demonstrated on MDCT and post-cholecystectomy bile leak revealed on Gd-EOB-DTPA (Primovist) enhanced MRCP. Eur J Radiol Extra 2010;73:e65-7. [Crossref]
- Vitellas KM, El-dieb A, Bennett WF, et al. Cholangiography with IV Mangafodipir Trisodium (Teslascan) to Evaluate Bile Duct Leaks After Study of 11 Patients. AJR Am J Roentgenol 2002;179:409-16. [Crossref] [PubMed]
- Massoumi H, Kiyici N, Hertan H. Bile Leak After Laparoscopic Cholecystectomy. J Clin Gastroenterol 2007;41:301-5. [Crossref] [PubMed]
- Bismuth H, Majno PE. Biliary strictures: Classification based on the principles of surgical treatment. World J Surg 2001;25:1241-4. [Crossref] [PubMed]
- Chun K. Recent classifications of the common bile duct injury. Korean J Hepatobiliary Pancreat Surg 2014;18:69-72. [Crossref] [PubMed]
- De Palma GD, Galloro G, Iuliano G, et al. Leaks from laparoscopic cholecystectomy. Hepatogastroenterology 2002;49:924-5. [PubMed]
- Schnelldorfer T, Sarr MG, Adams DB. What is the Duct of Luschka?-A Systematic Review. J Gastrointest Surg 2012;16:656-62. [Crossref] [PubMed]
- Seale MK, Catalano OA., Saini S, et al. Hepatobiliary-specific MR Contrast Agents: Role in Imaging the Liver and Biliary Tree. RadioGraphics 2009;29:1725-48. [Crossref] [PubMed]
- Palmucci S, Roccasalva F, Piccoli M, et al. Contrast-Enhanced Magnetic Resonance Cholangiography: Practical Tips and Clinical Indications for Biliary Disease Management. Gastroenterol Res Pract 2017;2017:2403012. [Crossref] [PubMed]
- Mergener K, Strobel JC, Suhocki P, et al. The role of ERCP in diagnosis and management of accessory bile duct leaks after cholecystectomy. Gastrointest Endosc 1999;50:527-31. [Crossref] [PubMed]
- Suhocki PV, Meyers WC. Injury to aberrant bile ducts during cholecystectomy: a common cause of diagnostic error and treatment delay. AJR Am J Roentgenol 1999;172:955-9. [Crossref] [PubMed]
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004;36:987-92. [Crossref] [PubMed]
- Wellner UF, Keck T. Leakage of Hepaticojejunal Anastomosis: Reoperation. Visc Med 2017;33:197-201. [Crossref] [PubMed]
- Antolovic D, Koch M, Galindo L, et al. Hepaticojejunostomy-analysis of risk factors for postoperative bile leaks and surgical complications. J Gastrointest Surg 2007;11:555-61. [Crossref] [PubMed]
- Boraschi P, Donati F. Biliary-enteric anastomoses: Spectrum of findings on Gd-EOB-DTPA-enhanced MR cholangiography. Abdom Imaging 2013;38:1351-9. [Crossref] [PubMed]
- Palmucci S. Focal liver lesions detection and characterization: The advantages of gadoxetic acid-enhanced liver MRI. World J Hepatol 2014;6:477-85. [Crossref] [PubMed]
- Nagle SK, Busse RF, Brau AC, et al. High resolution navigated three-dimensional T1-weighted hepatobiliary MRI using gadoxetic acid optimized for 1.5 Tesla. J Magn Reson Imaging 2012;36:890-9. [Crossref] [PubMed]
- Lee NK, Kim S, Lee JW, et al. Biliary {MR} imaging with Gd-EOB-DTPA and its clinical applications. Radiographics 2009;29:1707-24. [Crossref] [PubMed]
- Frydrychowicz A, Nagle SK, D’Souza SL, et al. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: a cross-over comparison of gadobenate dimeglumine and gadoxetic acid. J Magn Reson Imaging 2011;34:585-94. [Crossref] [PubMed]
- Stelter L, Grieser C, Fernándes CMP, et al. Flip angle modulations in late phase {Gd}-{EOB}-{DTPA} {MRI} improve the identification of the biliary system. Eur J Radiol 2012;81:e991-5. [Crossref] [PubMed]
- Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol 2004;14:559-78. [Crossref] [PubMed]
- Kim HY, Choi JY, Park CH, et al. Clinical factors predictive of insufficient liver enhancement on the hepatocyte-phase of Gd-EOB-DTPA-enhanced magnetic resonance imaging in patients with liver cirrhosis. J Gastroenterol 2013;48:1180-7. [Crossref] [PubMed]
- Motosugi U, Ichikawa T, Sano K, et al. Double-Dose Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Patients With Chronic Liver Disease. Invest Radiol 2011;46:141-5. [Crossref] [PubMed]
- Watanabe H, Kanematsu M, Goshima S, et al. Staging Hepatic Fibrosis: Comparison of Gadoxetate Disodium–enhanced and Diffusion-weighted MR Imaging—Preliminary Observations. Radiology 2011;259:142-50. [Crossref] [PubMed]
- Choi JY, Lee JM, Sirlin CB. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features. Radiology 2014;273:30-50. [Crossref] [PubMed]


