
Neuroimaging in emergency: a review of possible role of pineal gland disease
Introduction
The pineal gland region is a complex anatomical region where a spectrum of histologically different types of benign and malignant tumors can arise, and can also be affected by many entities seen more frequently elsewhere in the brain (1-10). The development of symptoms is due to the mass effect, and in some cases the onset can be acute, requiring prompt diagnosis and treatment. Computed tomography (11-15), and mostly magnetic resonance imaging (MRI) (16-21), play a key role for the instrumental diagnosis and in the interventional radiology setting, especially in the neuroradiological field (22-44), while limited applications are reserved to US and conventional radiographic examination (45-48). The purpose of this article is to review the normal and main pathologic findings in pineal gland diseases, with a particular focus on the neuroimaging of those pathologies causing acute symptomatology and that can be encountered in the emergency setting.
Normal pineal gland anatomy and function
The pineal gland (epiphysis) is a small structure (about 5 mm, weighing about 100 mg) located in the midline, above the tentorium and below the splenium of the corpus callosum. In approximately 40% of individuals, concentric calcifications are present within the pineal gland, although its significance is still not completely understood. The pineal gland is connected through the pineal stalk to the posterior roof of the third ventricle (10,49,50). Histologically, the normal pineal gland is composed by pineocytes (95%)—specialized neuron related to the retinal rods and cones—and astrocytes (5%), within a fibrovascular stroma. The pineal gland is not isolated by the blood-brain barrier, and therefore enhances after contrast medium administration (9).
One of the primary roles of the pineal gland is in the generation and regulation of biological rhythms producing melatonin (51); it is also implicated in the onset of puberty and reproductive functions.
Signs and symptoms of pineal gland pathology
Pathologic processes involving the pineal region produce signs and symptoms related to the mass effect on the adjacent tissues and invasion of surrounding structures. These include several acute symptoms, such as increased intracranial pressure syndrome from obstruction of the aqueduct of Sylvius and consequent hydrocephalus, and Parinaud syndrome. Parinaud syndrome is caused by the compression or invasion of the tectal plate and is characterized by supranuclear vertical gaze disturbance (often manifesting with diplopia), mydriasis, failed ocular convergence, and blepharospasm (52-54). As a result of increased intracranial pressure patients also present headache, nausea, and vomiting. Precocious puberty is a non-acute presentation, more commonly associated with germ cell tumors (GCTs), probably caused by an increased secretion of human chorionic gonadotropin (hCG). Pineal apoplexy is another acute presentation of pineal gland pathology, though rarer, and is caused by bleeding into a pineal tumor or cyst; the most common presenting symptom is a sudden decrease in consciousness associated with a headache. Secondary parkinsonism attributed to pineal lesions has also been reported (50).
Pineal gland diseases
Pineal gland tumors
Tumors of the pineal region can be histologically classified into those arising from the pineal parenchyma, germ cell neoplasms, and metastatic tumors (1,7,55,56). Pineal tumors are rare in adults, representing 0.4–1% of all brain tumors, while they occur in up to 3–8% in patients of pediatric age. Pineal tumors in children are usually also larger, due to greater extensibility and tissue plasticity (7).
Clinical presentation in the emergency setting: clinical presentation depends mostly on lesion size and localization. As other intracranial masses, pineal tumors can compress the aqueduct causing obstructive hydrocephalus and signs and symptoms of raised intracranial pressure. Parinaud syndrome due to pressure on the tectal plate can be another typical clinical presentation. Gait unsteadiness and ataxia have been described as a clinical presentation in pineal melanoma. Compression of the pituitary infundibulum could lead to diabetes insipidus (most common), hypopituitarism or optic chiasm compression with diplopia. When the thalami and basal ganglia are involved, the presentation is often delayed with a more massive tumor at diagnosis (56,57).
GCTs represent more than half of the pineal region tumors and are far more common in males. According to the WHO, they are classified into germinomas and non-germinomas. Nongerminomatous GCTs are represented by teratomas, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and the mixed GCTs (6). Most GCTs produce hormones and can be characterized serologically by increased serum and CSF levels of tumoral oncoproteins (α-fetoprotein, β-hCG, placental alkaline phosphatase). Germinomas are highly responsive to radiation therapy, and the overall prognosis is excellent, with a 5-year survival of about 90%.
Imaging findings: at computed tomography (CT) germinomas appear as sharply circumscribed, hyperattenuating mass (due to the highly cellular lymphocyte component) that typically engulfs pineal calcifications. MRI shows a solid mass that may have cystic components. Germinomas are iso- to hyperintense on T1- and T2-weighted images and show intense, homogeneous enhancement after gadolinium. Diffusion weighted imaging (DWI) may show restricted diffusion. The differential diagnosis is mainly with primary pineal neoplasms; however, the CT sign of engulfment of the pineal calcifications and the presence of elevated serum and CSF markers are crucial in narrowing the differential diagnosis (58). A metastatic epidural seeding of a pineal germinomas is not frequent but should be considered in the differential diagnosis of an enhanced epidural lesion (49). Tosaka et al. described a case of a 16-year-old boy, previously treated from a pineal germinoma, who developed rapidly progressing gait disturbances, with paraparesis and anesthesia of the L5–S1 territories on both sides, and urinary retention. Imaging evaluation revealed the presence of spinal epidural metastases, that required surgery in emergency and on pathologic examination were confirmed to be from germinoma (59).
Teratomas appear at imaging as multiloculated, lobulated lesions with intralesional areas of fat, calcifications, and fluid (2). On MRI, T2-weighted signal is iso- to hypointense in the soft tissue component, with enhancement on post-contrast images (2). Malignant counterparts of teratomas show a more homogeneous imaging appearance, with fewer cysts and calcifications, and should be included in the differential diagnosis with other pineal tumors. Secondary somatic malignancies are not rare, so surveillance for both secondary malignancy and growing teratoma syndrome are recommended.
Pineal parenchymal tumors are rare, representing less than 0.2% of intracranial neoplasms. They arise from the pineocytes and histologically are neuroepithelial neoplasms. Different tumoral grades are recognized, from the low-grade pineocytoma to the intermediate-grade pineal parenchymal tumor of intermediate differentiation (PPTID) and the highly malignant pineoblastoma.
Pineocytoma is a slow-growing tumor (grade I according to the WHO) and is one of the most common pineal parenchymal neoplasms. It mainly affects adults in the third and fourth decades without gender predilection. After radical surgery, there are no reported relapses, and the 5-year survival is 86–100%. Cerebrospinal fluid (CSF) seeding and metastases are also rare.
Pineoblastoma is a highly malignant (WHO grade IV) lesions, accounting for about 40% of pineal parenchymal tumors. It can occur at any age, but most frequently affects patients in the first two decades. CSF dissemination is frequent, and worsen the prognosis. The 5-year survival is less than 60%.
Imaging findings: pineocytomas appear as small (usually less than 3 cm), iso-hyperdense, well-circumscribed demarcated lesions at CT; unlike GCTs, pineal parenchymal tumors tend to expand and displace the normal pineal calcifications toward the periphery. MRI evaluation can better define internal tissue characteristics and vascularization after gadolinium administration (60-64). At MRI, pineocytomas are well-demarcated lesions, hypo-isointense on T1-weighted and hyperintense on T2-weighted sequences. After gadolinium, they typically show intense and homogeneous enhancement. Some pineocytomas may have cystic or partially cystic appearance, and the differential diagnosis with pineal cyst requires identification of internal or nodular wall enhancement. Intratumoral hemorrhage rarely occurs in pineocytomas.
Pinealoblastoma appears at CT as a larger (typically ≥3 cm), lobulated, hyperdense mass, with calcifications exploded at the periphery of the lesion. At MRI, pineoblastomas show heterogeneous signal intensity, with necrotic and hemorrhagic areas. DWI shows restricted diffusion. Cystic forms of pineoblastomas are rare, while CSF dissemination is a common finding, so imaging of the entire spine is recommended. At the time of the diagnosis, almost all patients have obstructive hydrocephalus and Parinaud syndrome A distinctive feature of metastatic pineoblastoma is that it secretes serotonin and can cause syndromes of neuroendocrine tumors, like circulation deficiency, myocardial ischemia, bronchoconstriction, venous thrombosis, and anasarca. Heithem et al. described a particularly rare case of acute circulatory deficiency due to massive serotonin release during surgical manipulation of an intra-abdominal metastasis pineoblastoma (5).
Pineal gland metastases: metastatic involvement of pineal gland is mostly due to the spread of primary carcinomas of the lung, breast, gastrointestinal tract, kidneys, bladder, pancreas, ovary. Malignant melanoma can occur in the pineal region as a primary or a metastatic tumor, though it was rarely described (55). Despite aggressive treatment strategies, the overall life expectancy of patients with metastatic melanoma is between 3 and 6 months (55-57,65).
Pineal metastases usually have a heterogeneous appearance, with hypointense signal on T1-W sequences. Approximately half of the melanoma metastases (melanotic type) are hyperintense on unenhanced T1-weighted images (56).
Pineal cysts
Imaging findings: pineal cysts appear at MRI as round or oval, thin-walled, and well-circumscribed lesions, with signal intensity similar to that of CSF (52), even if on fluid-attenuated inversion recovery (FLAIR) images, the signal may not be suppressed entirely due to the proteinaceous contents After gadolinium, enhancement of the cyst wall is typically incomplete (3,8,66-69) (Figure 1).
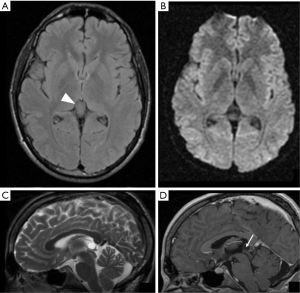
Clinical presentation in the emergency setting: the vast majority of pineal cysts are small (<1 cm) and asymptomatic and encountered incidentally on CT scans or MRI (3). Larger cysts can present with mass effect on the tectal plate leading to compression of the superior colliculi and Parinaud syndrome. If the cerebral aqueduct is compressed, they may also result in obstructive hydrocephalus. Sometimes symptoms can evolve rapidly and require prompt intervention. Tamura et al. (3) reported a case of a 61-year-old man, with a sudden onset of headache, reduced consciousness and diplopia in whom CT scans demonstrated a hyperdense pineal mass with dilatation of the lateral/third ventricles and intraventricular hemorrhage. The day after, the patient’s consciousness level declined, and CT scans demonstrated acute obstructive hydrocephalus which was emergently treated with external ventricular drainage.
Pineal apoplexy is a rare but acute clinical picture, and refers to a sudden neurological deterioration following hemorrhage in a pineal mass, most commonly into a pineal cyst (70,71). Patients develop a sudden severe headache, often with associated symptoms including decreased consciousness level and meningism. Hemorrhagic pineal cysts can occur at any age, from infants to senile patients. The grade and extent of hemorrhage can be variable, ranging from minor intracystic xanthochromic fluid levels to intraventricular hemorrhage. Some authors suggested a potentially increased risk of anticoagulation-induced hemorrhage in pineal cysts (3). These authors, reporting a case, suggest that it is advisable to inform patients with pineal cysts of the possible risk of intracystic hemorrhage and the potentially associated complication during anticoagulant or antiplatelet therapy (3). However, in the majority of reported cases to date, the exact cause of bleeding has not been completely understood. Intracystic hemorrhage presenting with new-onset seizures was also reported in the literature (6). More rarely, the underlying cause of bleeding is a cavernous angioma, as in the case described by Kobayashi et al. (72).
Sometimes apoplexy may follow an ischemic event in the context of a tumor. Indeed, large tumors may compress or outgrow the feeding vessels, especially those coming from the lateral pineal artery, which often provides unilateral vascularization to the pineal gland. An ischemic mechanism can be hypothesized when neuroimaging exams do not show signs of hemorrhage, as happened in a case of a vanishing pineal gland in a girl after acute onset of headaches, vomiting, dizziness, and tinnitus described by Patriarca et al. (73). In these cases, MRI is the modality of choice for follow-up examinations (74) (Figure 2). CNS lymphomas and other lesions are relatively frequently reported as vanishing tumors because of their trend to regress with corticosteroid therapy (69,75).
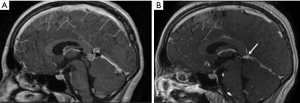
CNS lymphoma rarely involves the pineal gland, and few cases are reported in the literature (53). Headache is one of the most common presenting symptoms in these patients, although acute symptoms such as focal neurologic deficits, fever, diplopia, altered mental status, and seizure are also described. The average age at diagnosis is 40 years and is far more common in males. B-cell lymphoma is the most common type, including large B cell lymphoma, malignant B cell lymphoma, immunoblastic lymphoma, and anaplastic lymphoma kinase-positive anaplastic large cell lymphoma (ALK-1 positive ALCL) (53,54).
Imaging findings: imaging features of pineal lymphoma are not pathognomonic, overlapping with the appearance of pineoblastoma, germ cell tumor, and metastatic disease (53), so histologic confirmation is needed (76,77). On MRI lymphoma appears in most cases as a homogeneously enhancing lesion; hydrocephalus is often present at the time of the diagnosis.
Conclusions
Several pineal gland lesions manifest with an acute onset, the most common symptom being headache of sudden onset or acute worsening. Pineal apoplexy should always be considered in patients with a pineal cyst that become symptomatic. CT and MRI are valuable tools for the differential diagnosis, and in the emergency setting, prompt identification of pineal gland pathology is essential to improve survival of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abramson DH, Dunkel IJ, Marr BP, et al. Incidence of pineal gland cyst and pineoblastoma in children with retinoblastoma during the chemoreduction era. Am J Ophthalmol 2013;156:1319-20. [Crossref] [PubMed]
- De Los Reyes EVA, Rivera ID, Santos HM, et al. Mature teratoma of the pineal region in the paediatric age group : A case report and review of the literature. Malays J Pathol 2018;40:175-83. [PubMed]
- Tamura Y, Yamada Y, Tucker A, et al. Endoscopic Surgery for Hemorrhagic Pineal Cyst Following Antiplatelet Therapy: Case Report. Neurol Med Chir (Tokyo) 2013;53:625-9. [Crossref] [PubMed]
- Taraszewska A, Matyja E, Koszewski W, et al. Asymptomatic and symptomatic glial cysts of the pineal gland. Folia Neuropathol 2008;46:186-95. [PubMed]
- Heithem C, Issaoui G, Khadraoui M, et al. Acute circulatory deficiency due to endocrinal tumoral manipulation: The pinéaloblastoma. Pan Afr Med J 2014;18:168. [PubMed]
- Mehrzad R, Mishra S, Feinstein A, et al. A new identified complication of intracystic hemorrhage in a large pineal gland cyst. Clin Imaging 2014;38:515-7. [Crossref] [PubMed]
- Lensing FD, Abele TA, Sivakumar W, et al. Pineal Region Masses-Imaging Findings and Surgical Approaches. Curr Probl Diagn Radiol 2015;44:76-87. [Crossref] [PubMed]
- Whitehead MT, Oh CC, Choudhri AF. Incidental pineal cysts in children who undergo 3-T MRI. Pediatr Radiol 2013;43:1577-83. [Crossref] [PubMed]
- Smith AB, Rushing EJ, Smirniotopoulos JG. Lesions of the pineal region: radiologic-pathologic correlation. Radiographics 2010;30:2001-20. [Crossref] [PubMed]
- Korogi Y, Takahashi M, Ushio Y. MRI of pineal region tumors. J Neurooncol 2001;54:251-61. [Crossref] [PubMed]
- Cazzato RL, Arrigoni F, Boatta E, et al. Percutaneous management of bone metastases: state of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR). Radiol Med 2019;124:34-49. [Crossref] [PubMed]
- Di Cesare E, Patriarca L, Panebianco L, et al. Coronary computed tomography angiography in the evaluation of intermediate risk asymptomatic individuals. Radiol Med 2018;123:686-94. [Crossref] [PubMed]
- Barile A, Arrigoni F, Bruno F, et al. Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol 2018;14:2945-55. [Crossref] [PubMed]
- Arrigoni F, Bruno F, Zugaro L, et al. Role of interventional radiology in the management of musculoskeletal soft-tissue lesions. Radiol Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Arrigoni F, Bruno F, Zugaro L, et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed 2018;89:166-74. [PubMed]
- Michelini G, Corridore A, Torlone S, et al. Dynamic MRI in the evaluation of the spine: State of the art. Acta Biomed 2018;89:89-101. [PubMed]
- Bruno F, Barile A, Arrigoni F, et al. Weight-bearing MRI of the knee: A review of advantages and limits. Acta Biomed 2018;89:78-88. [PubMed]
- Masciocchi C, Zugaro L, Arrigoni F, et al. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol 2016;26:2472-81. [Crossref] [PubMed]
- Mariani S, La Marra A, Arrigoni F, et al. Dynamic measurement of patello-femoral joint alignment using weight-bearing magnetic resonance imaging (WB-MRI). Eur J Radiol 2015;84:2571-8. [Crossref] [PubMed]
- Di Cesare E, Cademartiri F, Carbone I, et al. Clinical indications for the use of cardiac MRI. By the SIRM Study Group on Cardiac Imaging. Radiol Med 2013;118:752-98. [Crossref] [PubMed]
- Di Cesare E, Splendiani A, Barile A, et al. CT and MR imaging of the thoracic aorta. Open Med (Wars) 2016;11:143-51. [Crossref] [PubMed]
- Di Cesare E, Puglielli E, Michelini O, et al. Malignant obstructive jaundice: comparison of MRCP and ERCP in the evaluation of distal lesions. Radiol Med 2003;105:445-53. [PubMed]
- Barile A, Regis G, Masi R, et al. Musculoskeletal tumours: preliminary experience with perfusion MRI. Radiol Med 2007;112:550-61. [Crossref] [PubMed]
- Barile A, Arrigoni F, Bruno F, et al. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am 2017;55:997-1007. [Crossref] [PubMed]
- Reginelli A, Zappia M, Barile A, et al. Strategies of imaging after orthopedic surgery. Musculoskelet Surg 2017;101:1. [Crossref] [PubMed]
- Barile A, Arrigoni F, Zugaro L, et al. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol 2017;34:53. [Crossref] [PubMed]
- Masciocchi C, Barile A, Lelli S, et al. Magnetic resonance imaging (MRI) and arthro-MRI in the evaluation of the chondral pathology of the knee joint. Radiol Med 2004;108:149-58. [PubMed]
- Barile A, Conti L, Lanni G, et al. Evaluation of medial meniscus tears and meniscal stability: Weight-bearing MRI vs arthroscopy. Eur J Radiol 2013;82:633-9. [Crossref] [PubMed]
- Barile A, Quarchioni S, Bruno F, et al. Interventional radiology of the thyroid gland: Critical review and state of the art. Gland Surg 2018;7:132-46. [Crossref] [PubMed]
- Barile A, Reginelli A, De Filippo M, et al. Diagnostic imaging and intervention of the musculoskeletal system: State of the art. Acta Biomed 2018;89:5-6. [PubMed]
- Zoccali C, Arrigoni F, Mariani S, et al. An unusual localization of chondroblastoma: The triradiate cartilage; from a case report a reconstructive technique proposal with imaging evolution. J Clin Orthop Trauma 2017;8:S48-52. [Crossref] [PubMed]
- Barile A, Bruno F, Arrigoni F, et al. Emergency and Trauma of the Ankle. Semin Musculoskelet Radiol 2017;21:282-9. [Crossref] [PubMed]
- Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol 2017;34:55. [Crossref] [PubMed]
- Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol 2017;34:52. [Crossref] [PubMed]
- Giordano AV, Arrigoni F, Bruno F, et al. Interventional Radiology Management of a Ruptured Lumbar Artery Pseudoaneurysm after Cryoablation and Vertebroplasty of a Lumbar Metastasis. Cardiovasc Intervent Radiol 2017;40:776-9. [Crossref] [PubMed]
- Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol 2016;89:20150355. [Crossref] [PubMed]
- Masciocchi C, Arrigoni F, La Marra A, et al. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol 2016;89:20150356. [Crossref] [PubMed]
- Ferrari F, Arrigoni F, Miccoli A, et al. Effectiveness of Magnetic Resonance-guided Focused Ultrasound Surgery (MRgFUS) in the uterine adenomyosis treatment: technical approach and MRI evaluation. Radiol Med 2016;121:153-61. [Crossref] [PubMed]
- Giacomelli R, Di Cesare E, Cipriani P, et al. Pharmacological stress, rest perfusion and delayed enhancement cardiac magnetic resonance identifies very early cardiac involvement in systemic sclerosis patients of recent onset. Int J Rheum Dis 2017;20:1247-60. [Crossref] [PubMed]
- Cappabianca S, Iaselli F, Reginelli A, et al. Value of diffusion-weighted magnetic resonance imaging in the characterization of complex adnexal masses. Tumori 2013;99:210-7. [Crossref] [PubMed]
- Jarre A, Llorens Salvador R, Montoliu Fornas G, et al. Value of brain MRI when sonography raises suspicion of agenesis of the corpus callosum in fetuses. Radiologia 2017;59:226-31. [Crossref] [PubMed]
- Di Cesare E, Gennarelli A, Di Sibio A, et al. Assessment of dose exposure and image quality in coronary angiography performed by 640-slice CT: a comparison between adaptive iterative and filtered back-projection algorithm by propensity analysis. Radiol Med 2014;119:642-9. [Crossref] [PubMed]
- Masciocchi C, Conti L, D’Orazio F, et al. Errors in musculoskeletal MRI. In: Errors in Radiology 2012:209-17.
- Dialetto G, Reginelli A, Cerrato M, et al. Endovascular stent-graft treatment of thoracic aortic syndromes: A 7-year experience. Eur J Radiol 2007;64:65-72. [Crossref] [PubMed]
- Mandato Y, Reginelli A, Galasso R, et al. Errors in the Radiological Evaluation of the Alimentary Tract: Part I. Semin Ultrasound CT MR 2012;33:300-7. [Crossref] [PubMed]
- Tamburrini S, Solazzo A, Sagnelli A, et al. Amyotrophic lateral sclerosis: sonographic evaluation of dysphagia. Radiol Med 2010;115:784-93. [Crossref] [PubMed]
- Perrotta FM, Astorri D, Zappia M, et al. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int 2016;36:1579-83. [Crossref] [PubMed]
- Cantisani V, Grazhdani H, Drakonaki E, et al. Strain US elastography for the characterization of thyroid nodules: Advantages and limitation. Int J Endocrinol 2015;2015:908575. [Crossref] [PubMed]
- Kim YH, Kim JW, Park CK, et al. Papillary tumor of pineal region presenting with leptomeningeal seeding. Neuropathology 2010;30:654-60. [Crossref] [PubMed]
- Dolendo MCJ, Lin TP, Tat OH, et al. Parkinsonism as an unusual presenting symptom of pineal gland teratoma. Pediatr Neurol 2003;28:310-2. [Crossref] [PubMed]
- Clark AR, Calligaris D, Regan MS, et al. Rapid discrimination of pediatric brain tumors by mass spectrometry imaging. J Neurooncol 2018;140:269-79. [Crossref] [PubMed]
- Bosnjak J, Budisic M, Azman D, et al. Pineal gland cysts--an overview. Acta Clin Croat 2009;48:355-8. [PubMed]
- Gupta A, Hussain A, Johnson M, et al. Pineal Gland Lymphoma: Case Report and Literature Review. J Clin Imaging Sci 2015;5:51. [Crossref] [PubMed]
- Yoshida T, Tezuka Y, Hirosawa T, et al. Pineal Malignant B-cell Lymphoma with Lower Cranial Nerve Involvement. Intern Med 2014;53:1205-8. [Crossref] [PubMed]
- Wendel C, Kaech DL, Woodtli M, et al. Primary Malignant Melanoma in the Pineal Region: Case Report and Literature Review. J Neurol Surg A Cent Eur Neurosurg 2018;79:344-52. [Crossref] [PubMed]
- Arlant PA, Grunnet ML, Heilbrun MP. Primary malignant melanoma of the pineal region. Surg Neurol 1977;7:121-3. [PubMed]
- Mitchell PJ, Funt SA, Gonzales MF, et al. Primary pineal and meningeal malignant melanomatosis. J Clin Neurosci 1998;5:353-6. [Crossref] [PubMed]
- Valentini G, Marcoccia A, Cuomo G, et al. Early systemic sclerosis: Marker autoantibodies and videocapillaroscopy patterns are each associated with distinct clinical, functional and cellular activation markers. Arthritis Res Ther 2013;15:R63. [Crossref] [PubMed]
- Tosaka M, Ogimi T, Itoh J, et al. Spinal epidural metastasis from pineal germinoma. Acta Neurochir (Wien) 2003;145:407-10; discussion 410. [PubMed]
- Gaudino S, Gangemi E, Colantonio R, et al. Neuroradiology of human prion diseases, diagnosis and differential diagnosis. Radiol Med 2017;122:369-85. [Crossref] [PubMed]
- Splendiani A, Perri M, Marsecano C, et al. Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol Med 2018;123:125-34. [Crossref] [PubMed]
- Splendiani A, Bruno F, Patriarca L, et al. Thoracic spine trauma: advanced imaging modality. Radiol Med 2016;121:780-92. [Crossref] [PubMed]
- Caranci F, Napoli M, Cirillo M, et al. Basilar artery hypoplasia. Neuroradiol J 2012;25:739-43. [Crossref] [PubMed]
- Caranci F, Briganti F, La Porta M, et al. Magnetic resonance imaging in brachial plexus injury. Musculoskelet Surg 2013;97 Suppl 2:S181-90. [Crossref] [PubMed]
- Martin-Blondel G, Rousseau A, Boch AL, et al. Primary pineal melanoma with leptomeningeal spreading: case report and review of the literature. Clin Neuropathol 2009;28:387-94. [PubMed]
- Costa F, Fornari M, Valla P, et al. Symptomatic pineal cyst: Case report and review of the literature. Minim Invasive Neurosurg 2008;51:231-3. [Crossref] [PubMed]
- Maurer PK, Ecklund J, Parisi JE, et al. Symptomatic pineal cyst: Case report. Neurosurgery 1990;27:451-3; discussion 453-4. [Crossref] [PubMed]
- Sarikaya-Seiwert S, Turowski B, Hänggi D, et al. Symptomatic intracystic hemorrhage in pineal cysts. J Neurosurg Pediatr 2009;4:130-6. [Crossref] [PubMed]
- Mattogno PP, Frassanito P, Massimi L, et al. Spontaneous Regression of Pineal Lesions: Ghost Tumor or Pineal Apoplexy? World Neurosurg 2016;88:64-9. [Crossref] [PubMed]
- Patel AJ, Fuller GN, Wildrick DM, et al. Pineal cyst apoplexy: Case report and review of the literature. Neurosurgery 2005;57:E1066. [Crossref] [PubMed]
- Osborn RE, Deen HG, Kerber CW, et al. A case of hemorrhagic pineal cyst: MR/CT correlation. Neuroradiology 1989;31:187-9. [Crossref] [PubMed]
- Kobayashi S, Kamagata M, Nakamura M, et al. Pineal apoplexy due to massive hemorrhage associated with cavernous angioma: Case report. Surg Neurol 2001;55:365-71. [Crossref] [PubMed]
- Patriarca L, D’Orazio F, Di Cesare E, et al. Vanishing pineal mass in a young patient without therapy: Case report and review of the literature. Neuroradiol J 2016;29:303-6. [Crossref] [PubMed]
- Bruno F, Smaldone F, Varrassi M, et al. MRI findings in lumbar spine following O2-O3 chemiodiscolysis: A long-term follow-up. Interv Neuroradiol 2017;23:444-50. [Crossref] [PubMed]
- Lucchetta M, Manara R, Perilongo G, et al. Regression of gadolinium-enhanced lesions in patients affected by neurofibromatosis type 1. Radiol Med 2016;121:214-7. [Crossref] [PubMed]
- Chini MG, Terracciano S, Riccio R, et al. Conformationally locked calixarene-based histone deacetylase inhibitors. Org Lett 2010;12:5382-5. [Crossref] [PubMed]
- Strocchia M, Terracciano S, Chini MG, et al. Targeting the Hsp90 C-terminal domain by the chemically accessible dihydropyrimidinone scaffold. Chem Commun (Camb) 2015;51:3850-3. [Crossref] [PubMed]


