
Bleeding complications after pancreatic surgery: interventional radiology management
Post-pancreatectomy hemorrhage
Epidemiology, classification and etiology
Pancreatoduodenectomy (PD) is known to be one of the most complex abdominal surgeries. Morbidity has decreased in the last years but is still reported between 30% and 40% (1,2), while peri-operative mortality at high volume centers is <3% (3,4). Post-pancreatectomy hemorrhage (PPH) is a relatively rare event, being reported in up to 10% of cases, but is responsible for 10–38% of mortality (5-14). The severity of this complication is confirmed by recent studies: patients with PPH showed more than a 6-fold increase in mortality when compared to those not affected, with 64% and 35% of them requiring one or multiple interventions, respectively (6).
The International Study Group for Pancreatic Surgery (ISGPS) created a classification based on bleeding onset (early if <24 h after surgery, delayed when >24 h), location (intraluminal or extraluminal), and severity (grade A, B, or C). PPH is classified as grade A when occurring early and having no major clinical impact; grade B includes early severe and delayed mild cases that require transfusions, intermediate care unit observation, or intervention; grade C PPH is a delayed, severe hemorrhage requiring intervention and should always be considered potentially life-threatening (15).
The distinction between early and delayed post-pancreatectomy hemorrhage (DPH) is crucial because they have different frequencies, etiologies and treatment strategies. DPH represent the majority of cases of PPH (14,16), with an incidence of 3.9% after pancreatic resection and a high mortality (between 30% and 50%) as reported by a metanalysis and a large series (17,18). The majority of DPH are arterial in origin (2,18); in a meta-analysis, pseudoaneurysms (PSA) were found to be involved in approximately one-third of cases (17). Regarding etiology, early hemorrhage is frequently a result of technical failure or vasospasm of small vessels on the surface of the pancreatic resection (16,19), while DPH has been widely associated to vessel erosion caused by anastomotic leakage including pancreatic fistula, infection, or intra-abdominal abscess (16-18,20-23). Among these causes, pancreatic fistula is the far most common. A meta-analysis reported intra-abdominal abscesses and/or anastomotic leaks in 65% of DPH patients (17) and recent studies have confirmed this strong association with a fistula being found in up to 80% of DPH cases (2,14,24). Clinically significant postoperative pancreatic fistula not only was the most uniformly associated and independent risk factor for the development of DPH (7,13,22,25,26), but also increased of 17-fold the bleeding-related mortality (12).
Clinical manifestation, diagnosis and management
While early PPH occurs by definition within 24 h from resection, DPH can occur many days and even weeks later, with a median onset reported of 10–27 days after surgery (10,17,27), a range as wide as 4–240 days post-operation (28), and a large part of events occurring after discharge from hospital (2,11). Intraluminal PPH is defined as the occurrence of blood draining from the nasogastric tube, hematemesis, or melena, and is frequently associated with ulcers at the anastomotic site or anastomotic fistulae possibly causing rupture of a PSA. Extraluminal PPH is defined as the occurrence of blood in drains or in an intra-abdominal location, and is frequently caused by pancreatic fistulae (17,29). However, if anastomotic dehiscence is present, extraluminal blood can present as intraluminal and vice versa (18,30). The most common initial presentation of delayed PPH has been reported to be intraluminal in nature by some authors (2,11) and extraluminal by others (17,18). A particular clinical presentation pattern of post-operative bleeding is known as Quincke’s triad, which is defined as the presence jaundice, biliary colic and signs of gastrointestinal hemorrhage; this entity occurs when a PSA of the hepatic artery ruptures into the biliary tree (31). Since up to 76% of the PSA involving the hepatic artery ultimately undergoes rupture and the mortality rate of such event ranges from 35% to 50%, an aggressive management is mandatory (32).
The initial sign of PPH can also be sentinel bleeding, defined as a self-limiting episode of bleeding in the form of blood loss from a drainage, hematemesis, melena, and/or a minor drop in hemoglobin count, with the patient remaining stable. This particular manifestation, corresponding to grade B delayed and mild PPH when applying the ISGPS classification system, is of outmost importance, since it implies a structural vascular defect and may precede the development of hemorrhagic shock (25,33,34). In fact, sentinel bleeding precedes a severe PPH in 50–80% of patients and thus requires immediate diagnostic workup (1,9,10,18,21,22,26,35). Some authors have suggested to perform an angiographic study for every sentinel bleeding after pancreatic surgery (34), but the source cannot be demonstrated in many cases, probably due to the intermittent character of hemorrhage (30).
When PPH occurs, decisions regarding the most diagnostic or therapeutic intervention are made on the basis of two main aspects: patient’s hemodynamic status and CT findings (22). In case of hemodynamic instability, regardless of the timing of bleeding, urgent laparotomy is usually indicated. Many authors routinely advocate immediate reoperation in every case of early PPH, avoiding poly-transfusions and any delay in treatment (22,36). In fact, early PPH is most commonly the result of incomplete hemostasis and thus can be effectively managed by surgical reintervention (18). When the patient is hemodynamically stable, it’s generally recommended to perform abdominal and pelvic multidetector computer tomography (MDCT) angiography as soon as sentinel bleeding is observed, when sensitivity is highest (12,22,29,37,38). MDCT angiography can show the cause, nature and site of bleeding, being a fundamental tool as it can avoid or guide treatment (10,15,39-41). Nonetheless, even if MDCT is performed, a clear bleeding site cannot be identified in many cases (1). This is especially true when imaging is delayed, unless a clear structural vascular abnormality or a PSA are the cause of bleeding (29). Although several studies are in favour of upper endoscopy when intraluminal hemorrhage is suspected (19), recent reports have shown that not only it may result inconclusive in the identification of the source of bleeding, but most importantly positive findings at endoscopic examination (for example erosive gastritis) may be dangerous as they can delay intervention, possibly causing death (16,17,42,43). Moreover, MDCT was recently proved to be superior to endoscopy in the diagnosis of upper intraluminal bleeding (44). When, after an initial diagnostic workup, the source of bleeding remains uncertain, it’s generally recommended to perform diagnostic angiography of the celiac axis and superior mesenteric artery. This procedure can demonstrate direct signs of bleeding, as active contrast extravasation, or indirect signs, like spastic and irregular vessels (29). Diagnostic angiography can be limited, however, in cases of diffuse, venous, or intermittent hemorrhage.
DPH represents to date a major cause of postoperative mortality; this complication is extremely difficult to manage because of the unstable clinical condition of the patients, that deteriorates rapidly (18,22). In the past, laparotomy has been considered as the treatment of choice for DPH (45). Surgical reintervention can achieve hemostasis but also has the advantage of allowing treatment of the factors associated with DPH, like fistulae or abdominal collections (17). Nonetheless, urgent re-look laparotomy is historically associated with high rates of morbidity and mortality, and surgery at this time is made challenging by postoperative adhesions and inflammation (10,46-48). The above-mentioned difficulties are often encountered when a pancreatic leak is present, and in such cases a first-line non-operative approach is given high priority by many authors (2,11,18,36). Moreover, patients with DPH are most frequently critically ill, so it has to be considered that general anesthesia and a long surgical procedure may worsen their clinical condition and increase their risk of death (30). These issues have pushed advances and research in less invasive techniques like the ones of interventional radiology (IR).
Arteriography with embolization is a well-known technique (20,43,49). IR is less invasive than surgery, doesn’t always need to be followed by Intensive Unit care, and allows a faster recovery and discharge (22). In the last years a number of studies have been reported about the management of DPH by IR, which has proven to be safe and effective, gaining more and more acceptance, and a clear shift toward its use has been widely documented (2,7,17-19,23,36,43,50,51). Today, endovascular treatment with percutaneous approach is recommended as the first choice by many authors in patients with DPH; exceptions are represented by cases in which adequate resuscitation cannot be achieved or in cases of logistic issues (7,10,14,15,17,22,29,33,37,52,53). The importance of the patient’s hemodynamic status was confirmed by a systematic review in which instability resulted the main cause for not performing angiography (10). Some authors advocate to always attempt the restoration of hemodynamic stability, even in critical cases, in order to allow endovascular intervention and avoid surgery; when surgical intervention is indeed performed on unstable and non-resuscitable patients, a deleterious outcome is frequently expected. The same authors conclude that IR is the best treatment for major visceral arterial bleeding when performed by experienced radiologists, and thus should be preferred over surgery whenever feasible (37). Nonetheless, there is no universally accepted treatment algorithm for patients with DPH, a well-recognized but also relatively uncommon, complication. It’s difficult to formulate firm recommendations, too (1,2,6,7,17). Several authors have tried to propose treatment algorithms but most of their studies are limited by small populations and low complication rates; the studies with larger populations, on the other hand, are characterized by prolonged eras (2,14,17,18,30). Some grade of inability in predicting the most suitable treatment for each patient still remains, with some authors concluding that the type of intervention requested in patients with DPH is unpredictable, sometimes even within a single institution (7,10,17,53). In a recent study a univariate analysis was performed to detect any predictors of which type of emergency intervention, surgical or radiological, the patient with DPH should initially receive, but no significant predictors were found (7).
Efficacy and roles of IR and surgery
Surgical treatment of post-pancreatectomy bleeding is associated with high rates of morbidity and mortality (46). A number of studies (Table 1) have analyzed the role of IR in patients with DPH, especially in cases where a PSA or major arterial bleeding were involved, and reported high success rates (36,37,54,55,62,63). In literature, technical success of endovascular treatment of DPH is reported between 82% and 100%; in the same studies recurrent bleeding is reported between 7% and 30%, hepatic complications between 12% and 63%, mortality between 7% and 54% (9,20,27,33,43,52,53). Several studies, including one systematic review, have reported a significantly lower mortality of IR when compared with surgical care (10,27,29,43). In the specific setting of visceral artery PSAs, which represent at least one third of causes of DPH (17), angiography was found to be associated with a fourth of the mortality of surgery, shorter operating time, lower blood loss, and shorter intensive care time, with an overall success rate at achieving hemostasis of 87% (56). A metanalysis analyzing 163 cases of DPH after PD found no statistically significant differences between IR and surgery in the rates of complete hemostasis achieved, complications or mortality; nonetheless, trends in favour of IR were observed in terms of complication rates (70% in surgical group vs. 36% in endovascular group) and mortality (43% vs. 21%) (17). A more recent systematic review did report a statistically significant difference in terms of mortality (22% for IR vs. 47% for laparotomy), a result that strongly supports the use of IR (10).
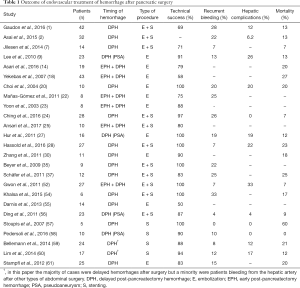
Full table
To our knowledge, no prospective randomized clinical trial comparing surgery and IR in the setting of DPH has been conducted to date, mostly because of the limited numbers of events; as a consequence, there is no evidence suggesting the superiority of one strategy over the other. The management is still ultimately decided on the basis of the characteristics and clinical status of the individual patient, together with the institutional preference and expertise. However, IR is widely considered as the potential first-line treatment in patients that are hemodynamically stable, while an aggressive surgical intervention is the preferred option in patients that are unstable or when others treatments have failed (17,28). Surgery continues to have an important role in the treatment of the causes of hemorrhage (as abdominal collections or fistulae) in patients where acute bleeding has been successfully managed by IR (37). It has to be pointed out that bleeding control must be followed by treatment of the underlying cause, most commonly represented by pancreatic fistula, either with IR or surgery, to ensure the best clinical outcomes for the patients. To avoid transportation it is desirable for the interventional and surgical team to have the possibility of working in close proximity to the place where the medical team is resuscitating. This can be achieved with the use of a hybrid operating room (64,65).
IR
Techniques and scenarios
Transcatheter arterial embolization is a well established, safe and effective treatment for ruptured PSA (59,61,66-68) as hemostasis is reached in 80% to 100% of patients (1). According to different papers, the gastroduodenal artery (GDA) stump is the most common bleeding site, followed by common hepatic artery (CHA), left hepatic artery, dorsal pancreatic artery, gastric artery, splenic artery (Figure 1), and the superior mesenteric artery, in different orders according to some different experiences(1,14,27,28,52,69). The majority of DPH are, according to the ISGPS classification, extraluminal (30). A range of different techniques are available to stop the hemorrhage once the bleeding source has been identified. When the target vessel is terminal in location, its proximal embolization is usually enough to stop the bleeding; on the other hand, if collaterals are present, both the inflow and outflow vessels have to be embolized (the so-called “sandwich” technique) in order to avoid re-bleeding (Figure 1) (70). Vascular occlusion can be obtained exploiting a wind range of materials, with the choice made primarily according to the desired type of vascular occlusion (transient or permanent). Transient embolization is reached using resorbable materials (i.e., gelatine or fibrin sponge), which allows for reperfusion of the treated area after a variable window of time. The main advantage is to avoid the definitive occlusion of the treated vessel. However, a non-negligible risk of rebleeding must be taken into account once the material has been absorbed. Permanent embolisation is reached using non-resorbable materials that induce a permanent vessel occlusion. To date, there is no consensus regarding an ideal embolizing agent; nevertheless, every case may need a specific embolic agent and the prompt availability of the material is required into any angio suite (41). The list of embolic materials includes polyvinyl alcohol, bucrilate, metallic coils or detachable balloons. Coils are suited when there is a single feeding vessel that can be sacrificed (Figure 2). Glue can be used to embolize small collaterals that cannot be directly catheterized. Balloon occlusion can be used as a temporary measure for protection of distal circulation.
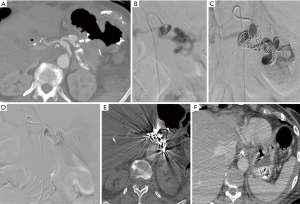
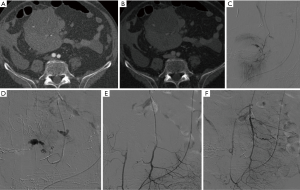
Embolization of GDA stump or CHA
Since the GDA stump and the CHA are the two most common bleeding sites, this review will focus more specifically in the treatment options of these vessels. Two techniques of embolization have been described: embolization of the hepatic artery proximal and distal to the GDA stump, or selective embolization of the GDA stump. Embolization of the hepatic artery is technically easy and usually allows a definite control of the bleeding, but it can lead to severe hepatic ischemic complications (71); infarction has been described in 30% to 66% of cases after using this technique in various studies (23,53) and therefore verification of portal vein (PV) patency is always mandatory before hepatic artery embolization (53,72). Selective embolization of the GDA stump allows to preserve hepatic arterial flow but is technically more complex and sometimes non-feasible, depending on the length of the stump and the morphology of the PSA, and is more prone to rebleeding, too (27,50,67,73). Hur et al. reported 100% rate of bleeding recurrence when selective embolization of the GDA stump was performed trying to spare the hepatic arterial flow; rebleeding was demonstrated to possibly occur not only at the GDA stump again, but also along any other portion of the extrahepatic segments of the hepatic arteries, especially when exposed to free pancreatic fluid. Whenever the bleeding is suspected to be associated with a pancreatic fistula, they therefore suggest to perform an aggressive embolization, comprehensive of the proper and common hepatic arteries (27). A PSA that is not yet excluded after embolization can be managed with percutaneous injection of thrombin under ultrasound or CT guidance (74).
Stent-grafts
In the last years, thanks to the advancements in technology and the wide commercial availability, an increasing number of papers have described the use of stent-grafts for endovascular repair of visceral aneurysms and PSAs (74). In the particular setting of a PPH, placing a stent-graft instead of using permanent embolic agents allows to maintain the hepato-portal blood flow and to reduce complications such as postembolization syndrome, liver abscess formation, and liver failure (28). For these reasons some authors have suggested that releasing a stent-graft into the hepatic artery, excluding the GDA stump, should be considered as a first-line procedure (9,75). According to some reports, covered stent-grafts are associated with a diminished risk of recurrent hemorrhage when compared to selective embolization (57,76). On the other hand, this technically challenging, especially in cases of difficult anatomy (9,27,52,72). The vascular access is generally performed via a transfemoral approach, but in case of marked angulation of the celiac axis or median arcuate ligament syndrome, which is associated with higher rates of technical failure and complications, some authors have suggested a brachial or transhepatic access (29,77,78). In an emergency setting, when time becomes crucial, stent-graft placement can lead to immediate exclusion of the target PSA with a relatively short operative time (28). Moreover, stent graft implantation has been associated with a lower use of fluoroscopy during the procedure (58). The most common complications of stent graft implantations are endoleaks and stent thromboses (58,79), but stent dislocation and consequent rebleedings have also been described (80). Another potential severe complication is infection of the stent when contact with gastrointestinal content or infected necrotic tissue occurs (58).
Currently, data over midterm and long-term stent patency are limited. Lim et al., in a mean follow-up of 356 days, reported a patency rate of 69% (60). Moreover, to date, there are no definite guidelines concerning the most appropriate anticoagulant therapy after implantation of arterial covered stents, and clinical experience is limited; nevertheless, after stent-graft implantation, an antiplatelet therapy is recommended. The general consensus is that clinically stable patients should receive acetylsalicylic acid 100 mg daily on a lifelong basis (28). The choice of the correct stent, both in diameter and length, can be difficult. First, it is important to avoid undersizing, taking into account that the diameter of the affected vessel, especially in an emergency setting, is often decreased as a result of hypovolemia and vascular spasms; undersizing can lead to inappropriate sealing, endoleaks and stent migration (28,59). On the other hand, stent oversizing can lead to vessel rupture, and is a probable cause of stent thrombosis (52). To date, limited data are available on the topic of stent sizing, although generally the angiographic findings and the preinterventional MDCT scans are key elements in the interventional radiologist’s choice (28). Proper stent length with correct landing zones is essential to avoid rebleeding, especially in patients with vessel erosion due to pancreatic or anastomotic leakage. Contraindication to stent-graft implantation can be short- or wide-neck aneurysm, and tortuous, stenotic or small vessels (28,81,82).
Complications
Percutaneous arterial embolization is a procedure at risk of recurrent hemorrhage with not negligible mortality rate (1). In a recent study rebleeding was seen in 25% of patients and it was due to recurrent PSAs, rebleeding from coiled vessels, blocked stents and endoleaks. Recurrence of bleeding is usually related to persistence of the favoring factors (i.e., pancreatic/bile leak, persistent abscess) (24). The GDA stump is the first spot to look for complications and surgical clips adjacent to the hepatic artery can aid in finding its position (29). Moreover, the patient’s outcome after the procedure can be worsened by hepatic complications such as the occurrence of postembolization liver failure or liver abscess (1). Septic shock, hemorrhagic shock, hepatic failure, and multiple organ failure are among the causes of death after IR in a meta-analysis, which occurred in 20% of treated patients (17).
In general, the liver can tolerate considerable amounts of embolic agents because of its multiple collateral pathways, mainly via subphrenic arteries and PV. As long as these vessels are proved to be normally present and patent, some authors suggest that embolization proximal to the proper hepatic artery should lead to a successful outcome (30). Nevertheless, the occlusion of the hepatic artery can lead to cholangitis, hepatic abscess, or fatal hepatic failure (17). Temporary elevation of blood liver enzymes, with normalization within a mean time of 12 days, has also been reported in case of embolization at the level of the GDA stump (52).
Other vascular complications
PV stenosis and portal hypertension
When PD is accompanied by intraoperative radiotherapy or venous reconstructions some studies report a 13–20% incidence of portomesenteric venous obstruction (83-86). Portal hypertension (PH) can cause ascites, abdominal bloating and pain, with a major impact on quality of life, but can also lead to life-threatening conditions, like variceal hemorrhage (87). The initial assessment in PV stenosis is initially performed by Doppler ultrasonography, followed by MDCT, or magnetic resonance angiography (88). Treatment options for PV stenosis vary from minimally invasive and palliative, like anticoagulation and paracentesis, to potentially curative but more aggressive and high-risk, such as open thrombectomy or portosystemic shunt creation. In these cases surgical approach is rarely chosen for various reasons: high morbidity and mortality rates, poor general condition of patients suffering from chronic disease, severe postoperative adhesions and high risk of bleeding from dilated collateral venous channels (69,87). High technical and clinical success rates, minimal invasiveness and low complication rates, have made the percutaneous endovascular approach the preferred treatment modality in the setting of PV stenosis (69). The first procedural step consists generally in venography, usually performed with percutaneous transhepatic approach. Transjugular, transsplenic, and intraoperative mesenteric vein approaches are also an option, with technical success rates reported between 66% and 100% (89,90). Recanalization of PV after PD (in some cases combined with intraoperative radiotherapy) can be achieved by the positioning of a stent with percutaneous approach; this can lead to symptomatic relief at the cost of minor morbidity and mortality in the majority of patients. However, stent patency and patient survival depend primarily on the course of the underlying oncologic disease; moreover, the small sample experiences found in literature can’t significantly support this treatment yet, also considering that the first reports of percutaneous recanalization of portomesenteric venous obstruction after PD showed a not negligible (29–50%) rate of restenosis that often required repeated intervention (91). In general, the positive outcome of endovascular PV recanalization seems to be related to the presence of tapering appearance of the patent vein, postoperative radiation treatment, length of occluded segment, malignant PV invasion, and presence of thrombotic component. Procedural complications are rare. These include hemothorax from the hepatic puncture site, pleural effusion, and unsatisfactory positioning of the stent (92).
Varices
With portal hypertension, splanchnic venous blood drains to the systemic system through collaterals and can lead to the development of varices in the duodenum, jejunum, or in the stomach. Among them, jejunal ectopic varices are the most commonly reported after hepatobiliary-pancreatic surgery. Collateral varices seem to form via low-resistant natural vascular spaces such as pancreaticoduodenal or gastrocolic veins along the afferent jejunal loop rather than newly formed postoperative tissue around the hepatic hilum (92). As a result, jejunal bleeding may happen. This event is rare and in literature only case reports or small series have reported it (93-96), as shown in Table 2. The two main options available are the treatment of the jejunal varices themselves or the decompression of the portal system via a trans-jugular portosystemic shunt; the choice between them generally depends on the clinical status of the patient. Direct treatment of the varices include their embolization via an endovascular approach or direct puncture technique, PV angioplasty and stenting, or both (94,97-100). In a series of 11 patients gastrointestinal bleeding from PV occlusion and jejunal varices occurred, on average, 27 months after surgery (92). Therefore, it is not always easy to correlate the two events and the diagnosis may be delayed until massive gastrointestinal bleeding from ectopic varix occurs (99). To our knowledge, the most delayed bleeding complication after PD occurred 2.5 years after surgery. A patient manifested signs of portal hypertension which caused him refractory melena. A gastroscopy revealed bleeding from the jejunal loop the varices couldn’t be treated via this approach. Eventually, partial splenic embolization with coils was carried out, achieving a ±50% area of splenic ischemia and successful hemostasis.
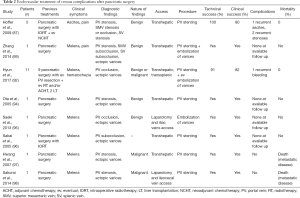
Full table
Conclusions
Vascular injuries after pancreatic surgery lead to dramatic consequences with high mortality and morbidity. Early recognition and treatment are essential to optimize the damage control and minimize complications and adverse events. Lately, IR intervention is becoming the preferred method to approach this condition because of a less invasivity and reduced mortality and several studies, including one systematic review, have reported a significantly lower mortality of IR when compared with surgical care. Transcatheter arterial embolization, in particular, has been shown to be a safe and effective first-line treatment for ruptured PSA occurring after PD. A range of different materials are nowadays available and the recent advancements in stent-graft technology have led to an increase use in this setting, with the potential to further reduce hepatic complications, such as postembolization syndrome, liver abscesses, and organ failure.
IR has been also gaining a more central role in the treatment of PV-related complications after pancreatic surgery such as PV stenosis, gastroesophageal and jejunal varices bleeding, with good success and relatively low adverse events.
Nevertheless, IR in this very specific field still remain a high-risk intervention with a significant percentage of recurrent hemorrhage and a high mortality rate. Therefore, that requires highly trained operators that could work in high-volume centers (101) and can establish a close and proficient collaboration with pancreatobiliary surgeons, anesthesiologists, and clinicians such hepatologists, oncologists and radiotherapists with the goal to improve the patient’s outcome and survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gaudon C, Soussan J, Louis G, et al. Late postpancreatectomy hemorrhage: Predictive factors of morbidity and mortality after percutaneous endovascular treatment. Diagn Interv Imaging 2016;97:1071-7. [Crossref] [PubMed]
- Asai K, Zaydfudim V, Truty M, et al. Management of a delayed post-pancreatoduodenectomy haemorrhage using endovascular techniques. HPB (Oxford) 2015;17:902-8. [Crossref] [PubMed]
- Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2013;15:49-60. [Crossref] [PubMed]
- He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB (OXFORD) 2014;16:83-90. [Crossref] [PubMed]
- Jagad RB, Koshariya M, Kawamoto J, et al. Postoperative hemorrhage after major pancreatobiliary surgery: an update. Hepatogastroenterology 2008;55:729-37. [PubMed]
- Kasumova GG, Eskander MF, Kent TS, et al. Hemorrhage after pancreaticoduodenectomy: does timing matter? HPB (Oxford) 2016;18:861-9. [Crossref] [PubMed]
- Jilesen AP, Tol JA, Busch OR, et al. Emergency management in patients with late hemorrhage after pancreatoduodenectomy for a periampullary tumor. World J Surg 2014;38:2438-47. [Crossref] [PubMed]
- Spiliopoulos S, Sabharwal T, Karnabatidis D, et al. Endovascular treatment of visceral aneurysms and pseudoaneurysms: Long-term outcomes from a Multicenter European study. Cardiovasc Intervent Radiol 2012;35:1315-25. [Crossref] [PubMed]
- Lee HG, Heo JS, Choi SH. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J Gastroenterol 2010;16:1239-44. [Crossref] [PubMed]
- Roulin D, Cerantola Y, Demartines N, et al. Systematic Review of Delayed Postoperative Hemorrhage after Pancreatic Resection. J Gastrointest Surg 2011;15:1055-62. [Crossref] [PubMed]
- Correa-Gallego C, Brennan MF, D’Angelica MI, et al. Contemporary experience with postpancreatectomy hemorrhage: results of 1,122 patients resected between 2006 and 2011. J Am Coll Surg 2012;215:616-21. [Crossref] [PubMed]
- Grützmann R, Rückert F, Hippe-Davies N, et al. Evaluation of the International Study Group of Pancreatic Surgery definition of post-pancreatectomy hemorrhage in a high-volume center. Surgery 2012;151:612-20. [Crossref] [PubMed]
- Wellner UF, Kulemann B, Lapshyn H, et al. Postpancreatectomy hemorrhage--incidence, treatment, and risk factors in over 1,000 pancreatic resections. J Gastrointest Surg 2014;18:464-75. [Crossref] [PubMed]
- Asari S, Matsumoto I, Toyama H, et al. Recommendation of treatment strategy for postpancreatectomy hemorrhage: lessons from a single-center experience in 35 patients. Pancreatology 2016;16:454-63. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH)-An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Rajarathinam G, Kannan DG, Vimalraj V, et al. Post pancreaticoduodenectomy haemorrhage: outcome prediction based on new ISGPS clinical severity grading. HPB (Oxford) 2008;10:363-70. [Crossref] [PubMed]
- Limongelli P, Khorsandi SE, Pai M, et al. Management of delayed postoperative hemorrhage after pancreaticoduodenectomy: a meta-analysis. Arch Surg 2008;143:1001-7. [Crossref] [PubMed]
- Yekebas EF, Wolfram L, Cataldegirmen G, et al. Postpancreatectomy hemorrhage: Diagnosis and treahage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 2007;246:269-80. [Crossref] [PubMed]
- Rumstadt B, Schwab M, Korth P, et al. Hemorrhage after pancreatoduodenectomy. Ann Surg 1998;227:236-41. [Crossref] [PubMed]
- Choi SH, Moon HJ, Heo JS, et al. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 2004;199:186-91. [Crossref] [PubMed]
- de Castro SM, Kuhlmann KF, Busch OR, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg 2005;241:85-91. [PubMed]
- Mañas-Gómez MJ, Rodríguez-Revuelto R, Balsells-Valls J, et al. Post-pancreaticoduodenectomy hemorrhage. Incidence, diagnosis, and treatment. World J Surg 2011;35:2543-8. [Crossref] [PubMed]
- Yoon YS, Kim SW, Her KH, et al. Management of postoperative hemorrhage after pancreatoduodenectomy. Hepatogastroenterology 2003;50:2208-12. [PubMed]
- Ching KC, Santos E, McCluskey KM, et al. Covered stents and coil embolization for treatment of postpancreatectomy arterial hemorrhage. J Vasc Interv Radiol 2016;27:73-9. [Crossref] [PubMed]
- Ansari D, Tingstedt B, Lindell G, et al. Hemorrhage after Major Pancreatic Resection: Incidence, Risk Factors, Management, and Outcome. Scand J Surg 2017;106:47-53. [Crossref] [PubMed]
- Ricci C, Casadei R, Buscemi S, et al. Late postpancreatectomy hemorrhage after pancreaticoduodenectomy: Is it possible to recognize risk factors? JOP 2012;13:193-8. [PubMed]
- Hur S, Yoon CJ, Kang SG, et al. Transcatheter arterial embolization of gastroduodenal artery stump pseudoaneurysms after pancreaticoduodenectomy: Safety and efficacy of two embolization techniques. J Vasc Interv Radiol 2011;22:294-301. [Crossref] [PubMed]
- Hassold N, Wolfschmidt F, Dierks A, et al. Effectiveness and outcome of endovascular therapy for late-onset postpancreatectomy hemorrhage using covered stents and embolization. J Vasc Surg 2016;64:1373-83. [Crossref] [PubMed]
- Puppala S, Patel J, McPherson S, et al. Hemorrhagic complications after whipple surgery: Imaging and radiologic intervention. AJR Am J Roentgenol 2011;196:192-7. [Crossref] [PubMed]
- Zhang J, Zhu X, Chen H, et al. Management of delayed post-pancreaticoduodenectomy arterial bleeding: Interventional radiological treatment First. Pancreatology 2011;11:455-63. [Crossref] [PubMed]
- Berceli SA. Hepatic and splenic artery aneurysms. Semin Vasc Surg 2005;18:196-201. [Crossref] [PubMed]
- Abbas MA, Fowl RJ, Stone WM, et al. Hepatic artery aneurysm: Factors that predict complications. J Vasc Surg 2003;38:41-5. [Crossref] [PubMed]
- Sato N, Yamaguchi K, Shimizu S, et al. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy. The importance of early angiography. Arch Surg 1998;133:1099-102. [Crossref] [PubMed]
- Tien YW, Wu YM, Liu KL, et al. Angiography is indicated for every sentinel bleed after pancreaticoduodenectomy. Ann Surg Oncol 2008;15:1855-61. [Crossref] [PubMed]
- Beyer L, Bonmardion R, Marciano S, et al. Results of non-operative therapy for delayed hemorrhage after pancreaticoduodenectomy. J Gastrointest Surg 2009;13:922-8. [Crossref] [PubMed]
- Blanc T, Cortes A, Goere D, et al. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg 2007;194:3-9. [Crossref] [PubMed]
- Schäfer M, Heinrich S, Pfammatter T, et al. Management of delayed major visceral arterial bleeding after pancreatic surgery. HPB (Oxford) 2011;13:132-8. [Crossref] [PubMed]
- Dohan A, Dautry R, Guerrache Y, et al. Three-dimensional MDCT angiography of splanchnic arteries: pearls and pitfalls. Diagn Interv Imaging 2015;96:187-200. [Crossref] [PubMed]
- Tonolini M, Ierardi AM, Carrafiello G. Elucidating early CT after pancreatico-duodenectomy: a primer for radiologists. Insights Imaging 2018;9:425-36. [Crossref] [PubMed]
- Smith SL, Hampson F, Duxbury M, et al. Computed tomography after radical pancreaticoduodenectomy (Whipple’s procedure). Clin Radiol 2008;63:921-8. [Crossref] [PubMed]
- Mauri G, Mattiuz C, Sconfienza LM, et al. Role of interventional radiology in the management of complications after pancreatic surgery: a pictorial review. Insights Imaging 2015;6:231-9. [Crossref] [PubMed]
- Tsirlis T, Vasiliades G, Koliopanos A, et al. Pancreatic leak related hemorrhage following pancreaticoduodenectomy. A case series. JOP 2009;10:492-5. [PubMed]
- Okuno A, Miyazaki M, Ito H, et al. Nonsurgical management of ruptured pseudoaneurysm in patients with hepatobiliary pancreatic diseases. Am J Gastroenterol 2001;96:1067-71. [Crossref] [PubMed]
- Frattaroli FM, Casciani E, Spoletini D, et al. Prospective study comparing multi-detector row CT and endoscopy in acute gastrointestinal bleeding. World J Surg 2009;33:2209-17. [Crossref] [PubMed]
- Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy. The 'sentinel bleed'. Arch Surg 1991;126:1037-40. [Crossref] [PubMed]
- Standop J, Glowka T, Schmitz V, et al. Operative re-intervention following pancreatic head resection: Indications and outcome. J Gastrointest Surg 2009;13:1503-9. [Crossref] [PubMed]
- Büchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 2003;138:1310-4; discussion 1315. [Crossref] [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected Adenocarcinoma of the Pancreas - 616 Patients: Results, Outcomes, and Prognostic Indicators. J Gastrointest Surg 2000;4:567-79. [Crossref] [PubMed]
- Khorsandi SE, Limongelli P, Jackson JE, et al. Management of delayed arterial hemorrhage after pancreaticoduodenectomy. A case series. JOP 2008;9:172-8. [PubMed]
- Otah E, Cushin BJ, Rozenblit GN. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg 2002;137:55-9. [Crossref] [PubMed]
- Mansueto G, D’Onofrio M, Iacono C, et al. Gastroduodenal artery stump haemorrhage following pylorus-sparing Whipple procedure: treatment with covered stents. Dig Surg 2002;19:237-40. [Crossref] [PubMed]
- Gwon DI, Ko GY, Sung KB, et al. Endovascular management of extrahepatic artery hemorrhage after pancreatobiliary surgery: clinical features and outcomes of transcatheter arterial embolization and stent-graft placement. AJR Am J Roentgenol 2011;196:W627-34. [Crossref] [PubMed]
- Miura F, Asano T, Amano H, et al. Management of postoperative arterial hemorrhage after pancreato-biliary surgery according to the site of bleeding: re-laparotomy or interventional radiology. J Hepatobiliary Pancreat Surg 2009;16:56-63. [Crossref] [PubMed]
- Khalsa BS, Imagawa DK, Chen JI, et al. Evolution in the treatment of delayed postpancreatectomy hemorrhage: Surgery to interventional radiology. Pancreas 2015;44:953-8. [Crossref] [PubMed]
- Darnis B, Lebeau R, Chopin-Laly X, et al. Postpancreatectomy hemorrhage (PPH): Predictors and management from a prospective database. Langenbecks Arch Surg 2013;398:441-8. [Crossref] [PubMed]
- Ding X, Zhu J, Zhu M, et al. Therapeutic Management of hemorrhage from visceral artery pseudoaneurysms after pancreatic surgery. J Gastrointest Surg 2011;15:1417-25. [Crossref] [PubMed]
- Stoupis C, Ludwig K, Inderbitzin D, et al. Stent grafting of acute hepatic artery bleeding following pancreatic head resection. Eur Radiol 2007;17:401-8. [Crossref] [PubMed]
- Pedersoli F, Isfort P, Keil S, et al. Stentgraft Implantation for the Treatment of Postoperative Hepatic Artery Pseudoaneurysm. Cardiovasc Intervent Radiol 2016;39:575-81. [Crossref] [PubMed]
- Bellemann N, Sommer CM, Mokry T, et al. Hepatic artery stent-grafts for the emergency treatment of acute bleeding. Eur J Radiol 2014;83:1799-803. [Crossref] [PubMed]
- Lim SJ, Park KB, Hyun DH, et al. Stent graft placement for postsurgical hemorrhage from the hepatic artery: clinical outcome and CT findings. J Vasc Interv Radiol 2014;25:1539-48. [Crossref] [PubMed]
- Stampfl U, Hackert T, Sommer CM, et al. Superselective embolization for the management of postpancreatectomy hemorrhage: A single-center experience in 25 patients. J Vasc Interv Radiol 2012;23:504-10. [Crossref] [PubMed]
- Robinson K, Rajebi MR, Zimmerman N, et al. Post-pancreaticoduodenectomy hemorrhage of unusual origin: treatment with endovascular embolization and the value of preoperative CT angiography. J Radiol Case Rep 2013;7:29-36. [PubMed]
- Ellison EC. Evidence-based management of hemorrhage after pancreaticoduodenectomy. Am J Surg 2007;194:10-2. [Crossref] [PubMed]
- Zhao DX, Leacche M, Balaguer JM, et al. Routine intraoperative completion angiography after coronary artery bypass grafting and 1-stop hybrid revascularization results from a fully integrated hybrid catheterization laboratory/operating room. J Am Coll Cardiol 2009;53:232-41. [Crossref] [PubMed]
- Byrne JG, Leacche M, Vaughan DE, et al. Hybrid cardiovascular procedures. JACC Cardiovasc Interv 2008;1:459-68. [Crossref] [PubMed]
- Flati G, Andrén-Sandberg Å, La Pinta M, et al. Potentially fatal bleeding in acute pancreatitis: Pathophysiology, prevention, and treatment. Pancreas 2003;26:8-14. [Crossref] [PubMed]
- Reber PU, Baer HU, Patel AG, et al. Superselective microcoil embolization: Treatment of choice in high-risk patients with extrahepatic pseudoaneurysms of the hepatic arteries. J Am Coll Surg 1998;186:325-30. [Crossref] [PubMed]
- Stampfl U, Sommer CM, Bellemann N, et al. The use of balloon-expandable stent grafts for the management of acute arterial bleeding. J Vasc Interv Radiol 2012;23:331-7. [Crossref] [PubMed]
- Zhang WG, Liu DM, Li Z, et al. Endovascular treatment for extrahepatic portal vein bifurcation stenosis after a whipple procedure using the kissing stents technique. Ann Vasc Surg 2014;28:264.e13-6. [Crossref] [PubMed]
- Rossi UG, Seitun S, Ferro C. Endovascular embolization of a third jejunal artery aneurysm: Isolation technique using the Amplatzer Vascular Plug 4. Catheter Cardiovasc Interv 2013;81:1049-52. [Crossref] [PubMed]
- Hackert T, Stampfl U, Schulz H, et al. Clinical significance of liver ischaemia after pancreatic resection. Br J Surg 2011;98:1760-5. [Crossref] [PubMed]
- Sato A, Yamada T, Takase K, et al. The fatal risk in hepatic artery embolization for hemostasis after pancreatic and hepatic surgery: Importance of collateral arterial pathways. J Vasc Interv Radiol 2011;22:287-93. [Crossref] [PubMed]
- Fujii Y, Shimada H, Endo I, et al. Management of massive arterial hemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg 2007;11:432-8. [Crossref] [PubMed]
- Wallace MJ, Choi E, McRae S, et al. Superior mesenteric artery pseudoaneurysm following pancreaticoduodenectomy: management by endovascular stent-graft placement and transluminal thrombin injection. Cardiovasc Intervent Radiol 2007;30:518-22. [Crossref] [PubMed]
- Makowiec F, Riediger H, Euringer W, et al. Management of delayed visceral arterial bleeding after pancreatic head resection. J Gastrointest Surg 2005;9:1293-9. [Crossref] [PubMed]
- Sanjay P, Kellner M, Tait IS. The role of interventional radiology in the management of surgical complications after pancreatoduodenectomy. HPB (Oxford) 2012;14:812-7. [Crossref] [PubMed]
- Papadopoulos P, Bize P, Guiu B, et al. Percutaneous transhepatic stent graft placement for treatment of hepatic artery injury after a Whipple procedure. J Vasc Interv Radiol 2014;25:977-8. [Crossref] [PubMed]
- Cassinotto C, Lapuyade B, Montaudon M. Two-way gastroduodenal artery. Diagn Interv Imaging 2013;94:330-2. [Crossref] [PubMed]
- Suzuki K, Mori Y, Komada T, et al. Stent-graft treatment for bleeding superior mesenteric artery pseudoaneurysm after pancreaticoduodenectomy. Cardiovasc Intervent Radiol 2009;32:762-6. [Crossref] [PubMed]
- Goltz JP, Bastürk P, Hoppe H, et al. Emergency and elective implantation of covered stent systems in iatrogenic arterial injuries. Rofo 2011;183:618-30. [Crossref] [PubMed]
- Belli AM, Markose G, Morgan R. The role of interventional radiology in the management of abdominal visceral artery aneurysms. Cardiovasc Intervent Radiol 2012;35:234-43. [Crossref] [PubMed]
- Claessen BE, Henriques JP, Jaffer FA, et al. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014;7:1081-92. [Crossref] [PubMed]
- Kang MJ, Jang JY, Chang YR, et al. Portal vein patency after pancreatoduodenectomy for periampullary cancer. Br J Surg 2015;102:77-84. [Crossref] [PubMed]
- Mitsunaga S, Kinoshita T, Kawashima M, et al. Extrahepatic portal vein occlusion without recurrence after pancreaticoduodenectomy and intraoperative radiation therapy. Int J Radiat Oncol Biol Phys 2006;64:730-5. [Crossref] [PubMed]
- Smoot RL, Christein JD, Farnell MB. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg 2006;10:1371-5. [Crossref] [PubMed]
- Shimizu Y, Yasui K, Fuwa N, et al. Late complication in patients undergoing pancreatic resection with intraoperative radiation therapy: gastrointestinal bleeding with occlusion of the portal system. J Gastroenterol Hepatol 2005;20:1235-40. [Crossref] [PubMed]
- Hoffer EK, Krohmer S, Gemery J, et al. Endovascular recanalization of symptomatic portomesenteric venous obstruction after pancreaticoduodenectomy and radiation. J Vasc Interv Radiol 2009;20:1633-7. [Crossref] [PubMed]
- Tajima T, Yoshimitsu K, Irie H, et al. Portal vein occlusion or stenosis in patients with hepatolithiasis: observation by multiphasic contrast-enhanced CT. Clin Radiol 2005;60:469-78. [Crossref] [PubMed]
- Cheng YF, Ou HY, Tsang LL, et al. Interventional percutaneous trans-splenic approach in the management of portal venous occlusion after living donor liver transplantation. Liver Transpl 2009;15:1378-80. [Crossref] [PubMed]
- Schneider N, Scanga A, Stokes L, et al. Portal vein stenosis: a rare yet clinically important cause of delayed-onset ascites after adult deceased donor liver transplantation: two case reports. Transplant Proc 2011;43:3829-34. [Crossref] [PubMed]
- Funaki B, Rosenblum JD, Leef JA, et al. Percutaneous treatment of portal venous stenosis in children and adolescents with segmental hepatic transplants: long-term results. Radiology 2000;215:147-51. [Crossref] [PubMed]
- Hyun D, Park KB, Cho SK, et al. Portal vein stenting for delayed jejunal varix bleeding associated with portal venous occlusion after hepatobiliary and pancreatic surgery. Korean J Radiol 2017;18:828-34. [Crossref] [PubMed]
- Choi JW, Kim HC, Jae HJ, et al. Transcatheter Embolotherapy with N-Butyl Cyanoacrylate for Ectopic Varices. Cardiovasc Intervent Radiol 2015;38:344-51. [Crossref] [PubMed]
- Ota S, Suzuki S, Mitsuoka H, et al. Effect of a portal venous stent for gastrointestinal hemorrhage from jejunal varices caused by portal hypertension after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg 2005;12:88-92. [Crossref] [PubMed]
- Saeki Y, Ide K, Kakizawa H, et al. Controlling the bleeding of jejunal varices formed at the site of choledochojejunostomy: report of 2 cases and a review of the literature. Surg Today 2013;43:550-5. [Crossref] [PubMed]
- Sakai M, Nakao A, Kaneko T, et al. Transhepatic portal venous angioplasty with stenting for bleeding jejunal varices. Hepatogastroenterology 2005;52:749-52. [PubMed]
- Hwang S, Sung KB, Park YH, et al. Portal vein stenting for portal hypertension caused by local recurrence after pancreatoduodenectomy for periampullary cancer. J Gastrointest Surg 2007;11:333-7. [Crossref] [PubMed]
- Sakurai K, Amano R, Yamamoto A, et al. Portal vein stenting to treat portal vein stenosis in a patient with malignant tumor and gastrointestinal bleeding. Int Surg 2014;99:91-5. [Crossref] [PubMed]
- Saad WE, Saad NE, Koizumi J. Stomal varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol 2013;16:176-84. [Crossref] [PubMed]
- Sasamoto A, Kamiya J, Nimura Y, et al. Successful embolization therapy for bleeding from jejunal varices after choledochojejunostomy: report of a case. Surg Today 2010;40:788-91. [Crossref] [PubMed]
- Lidsky ME, Sun Z, Nussbaum DP, et al. Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann Surg 2017;266:333-8. [Crossref] [PubMed]


