
Single stage, direct to implant pre-pectoral breast reconstruction
Introduction
While the breast is a pre-pectoral structure and breast restoration intuitively should occur in the same space, sub-pectoral breast reconstruction has been the mainstay of implant-based reconstruction for the last half century. While early implant-based breast reconstruction was attempted in the subcutaneous plane, device placement in the sub-pectoral location was favored to reduce the high rates of capsular contracture, infection, and implant loss seen after subcutaneous insertion (1-13). Sub-pectoral reconstruction continues to be in vogue to circumvent perceived concerns regarding implant visibility, palpability and failure.
Since the early days of implant-based breast reconstruction, the scientific evidence surrounding capsular contracture implicates bacterial contamination at time of implant insertion with biofilm formation (6,8,13). The advent of biofilm reduction techniques resulted in a significant reduction in capsular contracture rates, potentially minimizing one of the principal reasons for placing the implant beneath the muscle (14-16). Tissue expanders have been used routinely for two-stage breast reconstruction to recover skin domain lost after mastectomy since the late 1970s (9). However, as skin sparing and nipple sparing techniques became standard mastectomy approaches, the need for expansion has become somewhat redundant (17,18). Additionally, total sub-muscular coverage in the setting of expander-based breast reconstruction had been conceived to some extent to control expander position at the time of mastectomy. This was largely replaced by the introduction and subsequent adoption of lower pole coverage with acellular dermal matrices (ADM) (19,20). ADMs not only provided tissue reinforcement, but also better pocket control, and shape without the compressive effects of total sub-muscular coverage (21,22). Furthermore, expanders allow for controlled stepwise increases in volume, thereby reducing the risk of compressing the mastectomy flap’s delicate vascular supply. Immediate unsupported direct-to implant reconstructions with placement of the final prosthesis in the pre-pectoral position raise concerns regarding undue weight and tension on the mastectomy flap impeding tissue perfusion as well as subsequent thinning of the overlying mastectomy skin. ADM-reinforced direct-to-implant reconstruction offloads direct pressure on the mastectomy flaps with the weight being taken almost entirely by the ADM, mitigating these concerns. Moreover, perfusion assessment was, at best, an inexact science during the beginnings of implant-based breast reconstruction but has matured into a promising and reliable technology. Surgeons previously were reliant on clinical assessment and use of fluorescein. The advent of indocyanine green laser-induced fluorescence angiography was a turning point in mastectomy skin flap perfusion assessment and multispectral near infrared reflectance imaging is further improving perfusion assessment (23,24).
While two-stage sub-pectoral implant reconstruction gave reasonable results, it was apparent on critical appraisal, that patients experience significant physical limitations secondary to sub-pectoral implant placement. Most patients exhibit some degree of animation deformity during activity, particularly during adduction of the humerus (12,25-27). Sub-pectoral implant placement may also have more serious morbidity. Partial and complete loss of normal muscle fiber architecture has been documented when evaluating biopsies with electron microscopy after sub-pectoral tissue expansion and breast reconstruction (28). Additionally, although the evidence is conflicting and studies are limited in sample size, there are reports of significant reductions in function and strength amongst patients with sub-pectoral implants (25,29-34). Subcutaneous fat grafting has been used extensively in both surgeons’ practices (AK Antony, G Jones) in an effort to re-establish a gliding plane and ameliorate the disfigurement from animation deformity with limited success (35). Changing the position of reconstruction from the sub-pectoral to the pre-pectoral plane offers the opportunity to negate these effects, eliminating the distortion seen with sub-pectoral implant positioning (6,12,36-40). With the limitations imposed by sub-pectoral breast reconstruction, and the confluence of scientific achievement, improvement in surgical technique and advances in technology, successful pre-pectoral direct to implant breast reconstruction has become a reality.
Methods
Surgical technique
After completion of the mastectomy, the key to deciding to proceed with single stage reconstruction is based entirely on adequacy of mastectomy skin flap perfusion with a temporary sizer in place. In our collective experience, intraoperative skin flap perfusion is of primary importance in decision-making and supplants skin flap thickness. Fat grafting can be performed later as an adjunctive procedure to augment the mastectomy flaps if needed. Perfusion assessment is determined using either multispectral near infrared imaging with the Kent KD203 handheld device (Kent Medical Imaging) (G Jones), or, indocyanine green dye laser-induced fluorescence imaging (SPY, Stryker) (AK Antony). If skin perfusion is adequate with the appropriate temporary sizer in place, a decision is made to proceed with single stage direct-to-implant reconstruction in the pre-pectoral plane. If perfusion is marginal, an under-filled expander can be inserted, or the reconstruction delayed.
Both surgeons prefer an anterior tenting technique (G Jones, AK Antony). If skin perfusion is adequate, the mastectomy pocket and skin is prepared with betadine solution (16,41) A sheet of 16 cm × 20 cm thick ADM (AlloDerm, Allergan Inc.) is prepared according to manufacturer protocol and is sutured to the anterior surface of pectoralis major using the anterior tenting approach (42-44). Using 2-0 PDS, suturing is performed from 12 to 5 o’clock and 12 to 7 o’clock leaving an inferior access window for implant insertion (G Jones). Alternatively, interrupted 2-0 vicryl sutures can be placed to anchor the ADM and then 2-0 PDS is sutured to reinforce the medial and lateral border, again leaving an inferior window for access (AK Antony). The prepared pocket is re-checked with a sizer in place to ensure correct shape and position of the reconstruction. The sizer is removed and the pocket and chest are re-prepped with betadine or chlorhexidine/alcohol solution. The surgeon changes gloves and the implant is inserted using a Keller funnel to ensure no contact between the implant and the skin in an effort to minimize biofilm formation (15,16,45). The inferior ADM pocket is closed with 2-0 PDS sutured to the chest wall in a gentle curve to shape the inframammary fold. Closure of the skin is performed over 1–2 channel drains (G Jones) or 2 channel drains, one placed in the axilla and the other around the construct (AK Antony). AK Antony has implemented a strict drain protocol with maintenance of the drain until drainage is less than 20–25 cc/daily for 2 days; 1st drain removal typically occurs at post-operative day (POD) 10 and two ipsilateral drains are never removed simultaneously. Tegaderm occlusive dressing is used to cast the skin to optimize skin and nipple position and reduce shearing forces between the skin and ADM.
Results
Jones outcomes
One hundred and ninety-four breasts in 140 patients were operated upon with longest follow-up of 3.8 years. Successful outcome was achieved in 93.3% of cases (Figure 1). The most common complications were minor contour deformities at 44.3%, seromas 5.2%, and cellulitis in 5.7% patients. Explantation for any reason occurred in 6.7% of cases. There were no major full thickness skin necrosis requiring debridement and closure in the operating room. Partial thickness cutaneous blistering was treated conservatively with topical therapy in 4.1%. Minor rippling was present in 15% of cases and fat grafting was performed to soften minor contour deformities in 38%. There was 0% capsular contracture in non-radiated patients and 0% animation deformity. Long-term revision for implant size change occurred in 7.2% of cases.
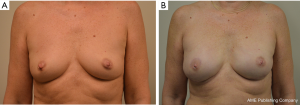
Antony outcomes
One hundred and sixty-three pre-pectoral reconstructions were carried out in 94 patients; of these, 111 (68%) were pre-pectoral direct to implant breast reconstructions (PP DTI). Longest follow-up was 3.6 years (mean follow-up of 15.1 months). Pre-pectoral tissue expander reconstruction was performed in lieu of direct-to-implant reconstruction for patient preference (the patient preferred to be more involved in the expansion process and determination of the final volume achieved) or if substantial size increase was planned in a smaller breasted patient. Successful outcome was achieved in 100% of PP DTI cases (Figure 2). The most common complications were minor contour deformity treated with fat grafting (13.5%), downsizing to TE with subsequent exchange for permanent implant (1.8%), hematoma (0.9%), and capsular contracture (0.9%). There were no seromas (0%), infections (0%), or device loss (0%). Revision for implant size change occurred in 1.8% of cases. Animation deformity occurred in 0% of cases.
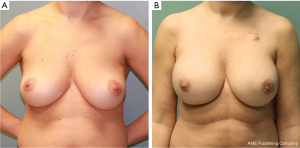
Discussion
Renewed interest in pre-pectoral reconstruction has emerged since Sigalove et al. first presented their amalgamated results of 353 reconstructions in 207 patients, of which 89% were two-stage pre-pectoral reconstruction (35). Pre-pectoral breast reconstruction remains predominantly tissue expander based (35,46-53). There is a rising interest in single stage, direct-to-implant pre-pectoral reconstruction and recent studies report favorable data on this state-of-the-art breast reconstruction modality (54-57). However, direct to implant breast reconstruction remains a smaller fraction (less than 15%) of implant-based reconstruction likely for concerns of high revision rates and the steep learning curve (58-60).
Direct-to-implant reconstruction has become more viable as a reconstructive option with modern mastectomy techniques that preserve ever increasing amounts of skin, fully realized with nipple sparing mastectomy (17,18). Two stage breast reconstruction was conceived when significant skin resection was carried out at the time of mastectomy. Expansion was the preferred method to restore skin surface area for the insertion of an adequate volume implant. As newer skin- and nipple-preserving mastectomy approaches have been adopted, the need for expansion has exponentially decreased. Additionally, the release of the fascial constraints of the breast at mastectomy often results in the breast skin envelope being capable of accommodating a similar, or even larger, sized implant than the original breast volume.
Insertion of a definitive implant in one stage is predicated entirely on adequate skin perfusion to maintain tissue viability. Many surgeons express concerns about the thickness of skin flaps presented to them by oncologic surgeons. It is often assumed that skin thickness is the primary determinant of viability. However, our experience with perfusion assessment devices demonstrates that thin post-mastectomy skin flaps are typically well perfused even when fully extended after the primary implant insertion has occurred at the time of mastectomy. The eligibility of a patient for pre-pectoral reconstruction is based on skin perfusion, rather than flap thickness, and objective assessment with a tissue perfusion system is of critical importance to achieving successful outcomes (49). Pre-pectoral reconstruction has been safely performed in the setting of thin mastectomy flaps, provided flap vascularity is maintained (46). In correlating risk factors with outcomes, patient characteristics that compromise skin flap vascularity such as smoking, uncontrolled diabetes and radiation proved to be the most significant factors contributing to complications after the procedure (13,49). We feel it is safe to proceed if adequate skin perfusion is demonstrated with the temporary sizer in place and consider single stage, pre-pectoral direct to implant reconstruction to be a safe, reproducible technique.
Both surgeons were routinely performing direct-to-implant reconstruction in the sub-pectoral position before converting to pre-pectoral direct to implant reconstruction. While limited studies comparing pre-pectoral and sub-pectoral DTI reconstruction are available, these have been positive. Antony et al found in a comparison of 134 DTI reconstructions that transitioning to pre-pectoral direct to implant did not result in increased complications, degradation of aesthetic results (aesthetic blinded panel evaluation favored PP reconstructions) or an increase in revisional procedures (43). Cattelani et al. evaluated pre-pectoral breast reconstruction (pre-pectoral ADM-assisted direct to implant compared with submuscular direct to implant and two-stage TE/I) and found less postoperative pain, faster recovery from postoperative upper extremity functional morbidity, higher aesthetic BREAST-Q scores as well as economic advantages in their series of 86 patients (57). Walia and colleagues also report significantly decreased postoperative pain in two stage pre-pectoral breast reconstruction patients without significant differences in BREAST-Q survey patient reported outcomes. However, there was a statistically significant increase in nipple ischemia amongst pre-pectoral patients (51). Finally, Baker et al. reported no significant difference in pain scores, early complications, or postoperative length of stay between direct to implant pre- and sub-pectoral breast reconstruction groups. However, more patients were dissatisfied with the amount of implant rippling in the pre-pectoral group (53).
At least three groups have looked at pre-pectoral breast reconstruction after post-mastectomy radiation therapy (PMRT) (55,56,61). Both Sigalove et al. and Elswick et al. found no statistically significant increased risk of adverse outcomes in pre-pectoral breast reconstructions that underwent PMRT based on short-term, retrospective data in 93 and 52 breasts respectively (55,61). More interesting is the results that follow from the study published by Sinnot and colleagues. These researchers found patients who underwent sub-pectoral breast reconstruction and receive PMRT actually had a greater rate of capsular contracture than patients that underwent pre-pectoral reconstruction (56).
The benefits of this technique include less patient discomfort, no need for post-operative expansion, less tissue flap edema, and virtually no subjective negative impact on upper extremity function. Additionally, animation deformity has been completely eliminated. Interestingly, fat grafting is required less frequently in our pre-pectoral patients compared to our sub-pectoral patients. This decreased rate of fat grafting is likely secondary to the elimination of animation deformity in pre-pectoral patients, and improved control over the medial aspect of the construct. Rippling perpendicular to the contraction of the pectoralis major muscle, and limitations in medial cleavage by the pectoralis major muscle are no longer an issue with pre-pectoral implant placement. The authors have been encouraged by the benefits seen from implementation of pre-pectoral direct to implant breast reconstruction in our practices and now routinely offer this modality to our patients as a primary reconstructive method.
Conclusions
Given the current trends in skin preservation during mastectomy, improved biofilm reduction algorithms, and advancements in tissue bioengineering and perfusion assessment, ADM-reinforced single stage, direct-to-implant insertion in the pre-pectoral space has become a viable alternative to two-stage expander-based, sub-pectoral reconstruction. We have experienced superior clinical and functional outcomes with minimal pain and enhanced convenience for the patient. Longer-term follow-up demonstrates maintenance of the integrity and quality of the reconstructions over time with extremely low rates of capsular contracture and complete absence of animation deformity. It is now the authors’ primary choice for immediate implant-based reconstruction following mastectomy.
Acknowledgements
We wish to thank Victor King MD, Aran Yoo MD, and Emilie Robinson MD for their assistance in preparing the data for portions of this text.
Footnote
Conflicts of Interest: G Jones is a consultant for Allergan Medical. AK Antony is a consultant for Allergan Medical Inc. and Stryker Inc.
Ethical Statement: The study was approved by the Institutional Review Board of Rush University Medical Center (No. 16071402) and the University of Illinois College of Medicine at Peoria.
References
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Berens JJ, Stapley LA. Breast tumors treated by mastectomy (subcutaneous) with mammary replacement. Ariz Med 1969;26:651-7. [PubMed]
- Hueston J, McKenzie G. Breast reconstruction after radical mastectomy. Aust N Z J Surg 1970;39:367-70. [Crossref] [PubMed]
- Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565-7. [Crossref] [PubMed]
- Guthrie RH. Breast reconstruction after radical mastectomy. Plast Reconstr Surg 1976;57:14-22. [Crossref] [PubMed]
- Schlenker JD, Bueno RA, Ricketson G, et al. Loss of Silicone Implants after Subcutaneous Mastectomy and Reconstruction. Plast Reconstr Surg 1978;62:853-61. [Crossref] [PubMed]
- Blevins PK. Subcutaneous mastectomy and breast replacement: its role in the treatment of benign, premalignant, and malignant breast disease. Am Surg 1981;47:281-6. [PubMed]
- Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312-7. [Crossref] [PubMed]
- Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982;69:195-208. [Crossref] [PubMed]
- Giraud B, Dauplat J, Gadonneix P, et al. Subcutaneous mammectomy with prosthetic inclusion. Apropos of 114 cases. Chirurgie 1986;112:402-12. [PubMed]
- Scarfì A, Ordemann K, Hüter J. Reconstruction of an ablated breast. Eur J Gynaecol Oncol 1986;7:93-6. [PubMed]
- Artz JS, Dinner MI, Sampliner J. Breast reconstruction with a subcutaneous tissue expander followed with a polyurethane-covered silicone breast implant. Ann Plast Surg 1988;20:517-21. [Crossref] [PubMed]
- Artz JS, Dinner MI, Foglietti MA, et al. Breast reconstruction utilizing subcutaneous tissue expansion followed by polyurethane-covered silicone implants: a 6-year experience. Plast Reconstr Surg 1991;88:635-9; discussion 640-1. [Crossref] [PubMed]
- Ajdic D, Zoghbi Y, Gerth D, et al. The Relationship of Bacterial Biofilms and Capsular Contracture in Breast Implants. Aesthet Surg J 2016;36:297-309. [Crossref] [PubMed]
- Deva AK, Adams WP Jr, Vickery K. The Role of Bacterial Biofilms in Device-Associated Infection. Plast Reconstr Surg 2013;132:1319-28. [Crossref] [PubMed]
- Jewell ML, Adams WP Jr. Betadine and Breast Implants. Aesthet Surg J 2018;38:623-6. [Crossref] [PubMed]
- Toth BA, Lappert P. Modified Skin Incisions for Mastectomy: The Need for Plastic Surgeons Input. Plast Reconstr Surg 1991;87:1048-53. [Crossref] [PubMed]
- Bishop CC, Singh S, Nash AG. Mastectomy and breast reconstruction preserving the nipple. Ann R Coll Surg Engl 1990;72:87-9. [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 2007;59:250-5. [Crossref] [PubMed]
- Duncan DI. Correction of implant rippling using allograft dermis. Aesthet Surg J 2001;21:81-4. [Crossref] [PubMed]
- Baxter RA. Intracapsular Allogenic Dermal Grafts for Breast Implant-Related Problems. Plast Reconstr Surg 2003;112:1692-6. [Crossref] [PubMed]
- Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [Crossref] [PubMed]
- Gurtner GC, Jones GE, Neligan PC, et al. Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 2013;7:1. [Crossref] [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- Becker H, Fregosi N. The Impact of Animation Deformity on Quality of Life in Post-Mastectomy Reconstruction Patients. Aesthet Surg J 2017;37:531-6. [Crossref] [PubMed]
- Nigro LC, Blanchet NP. Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1407. [Crossref] [PubMed]
- Gur E, Hanna W, Andrighetti L, et al. Light and electron microscopic evaluation of the pectoralis major muscle following tissue expansion for breast reconstruction. Plast Reconstr Surg 1998;102:1046-51. [Crossref] [PubMed]
- Banbury J, Yetman R, Lucas A, et al. Prospective Analysis of the Outcome of Subpectoral Breast Augmentation: Sensory Changes, Muscle Function, and Body Image. Plast Reconstr Surg 2004;113:701-7. [Crossref] [PubMed]
- Beals SP, Golden KA, Basten M, et al. Strength performance of the pectoralis major muscle after subpectoral breast augmentation surgery. Aesthet Surg J 2003;23:92-7. [Crossref] [PubMed]
- Becker H, Lind JG 2nd, Hopkins EG. Immediate Implant-based Prepectoral Breast Reconstruction Using a Vertical Incision. Plast Reconstr Surg Glob Open 2015;3:e412. [Crossref] [PubMed]
- de Haan A, Toor A, Hage JJ, et al. Function of the Pectoralis Major Muscle After Combined Skin-Sparing Mastectomy and Immediate Reconstruction by Subpectoral Implantation of a Prosthesis. Ann Plast Surg 2007;59:605-10. [Crossref] [PubMed]
- Hage JJ, van der Heeden JF, Lankhorst KM, et al. Impact of Combined Skin Sparing Mastectomy and Immediate Subpectoral Prosthetic Reconstruction on the Pectoralis Major Muscle Function. Ann Plast Surg 2014;72:631-7. [Crossref] [PubMed]
- Sarbak JM, Baker J. Effects of breast augmentation on pectoralis major muscle function in the athletic woman. Aesthet Surg J 2004;24:224-8. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Maxwell GP, Tornambe R. Management of mammary subpectoral implant distortion. Clin Plast Surg 1988;15:601-11. [PubMed]
- Hammond DC, Schmitt WP, O’Connor EA. Treatment of Breast Animation Deformity in Implant-Based Reconstruction with Pocket Change to the Subcutaneous Position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- Lesavoy MA, Trussler AP, Dickinson BP. Difficulties with Subpectoral Augmentation Mammaplasty and Its Correction: The Role of Subglandular Site Change in Revision Aesthetic Breast Surgery. Plast Reconstr Surg 2010;125:363-71. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:519-26. [Crossref] [PubMed]
- Hammond DC. Commentary on: Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:527-8. [Crossref] [PubMed]
- Campbell CA. The Role of Triple-Antibiotic Saline Irrigation in Breast Implant Surgery. Ann Plast Surg 2018;80:S398-402. [Crossref] [PubMed]
- Jones G, Yoo A, King V, et al. Prepectoral Immediate Direct-to-Implant Breast Reconstruction with Anterior AlloDerm Coverage. Plast Reconstr Surg 2017;140:31S-8S. [Crossref] [PubMed]
- Antony AK, Poirier J, Madrigrano A, et al. Evolution of the Surgical Technique for “Breast in a Day” Direct to Implant Breast Reconstruction: Transitioning from Dual Plane to Pre-Pectoral Implant Placement. Plast Reconstr Surg 2018. In press.
- Antony AK, Robinson EC. An Algorithmic Approach to Prepectoral Direct-to-Implant Breast Reconstruction: Version 2.0. Plast Reconstr Surg 2018. In press.
- Flugstad NA, Pozner JN, Baxter RA, et al. Does Implant Insertion with a Funnel Decrease Capsular Contracture? A Preliminary Report. Aesthet Surg J 2016;36:550-6. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Vidya R. Prepectoral Breast Reconstruction or Muscle-Sparing Technique with the Braxon Porcine Acellular Dermal Matrix. Plast Reconstr Surg Glob Open 2017;5:e1364. [Crossref] [PubMed]
- Gabriel A, Maxwell GP. Prepectoral Breast Reconstruction in Challenging Patients. Plast Reconstr Surg 2017;140:14S-21S. [Crossref] [PubMed]
- Paydar KZ, Wirth GA, Mowlds DS. Prepectoral Breast Reconstruction with Fenestrated Acellular Dermal Matrix. Plast Reconstr Surg Glob Open 2018;6:e1712. [Crossref] [PubMed]
- Walia GS, Aston J, Bello R, et al. Prepectoral Versus Subpectoral Tissue Expander Placement. Plast Reconstr Surg Glob Open 2018;6:e1731-6. [Crossref] [PubMed]
- Pittman TA, Abbate OA, Economides JM. The P1 Method: Prepectoral Breast Reconstruction to Minimize the Palpable Implant Edge and Upper Pole Rippling. Ann Plast Surg 2018;80:487-92. [PubMed]
- Baker BG, Irri R, MacCallum V, et al. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast Reconstr Surg 2018;141:1077-84. [Crossref] [PubMed]
- Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon®. Gland Surg 2017;6:682-8. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]
- Cattelani L, Polotto S, Arcuri MF, et al. One-Step Prepectoral Breast Reconstruction With Dermal Matrix-Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clin Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]
- American Society of Plastic Surgeons. 2017 Reconstructive Breast Procedures. 2017. American Society of Plastic Surgeons, Arlington Heights, IL. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf
- Clarke-Pearson EM, Lin AM, Hertl C, et al. Revisions in implant-based breast reconstruction: how does direct-to-implant measure up? Plast Reconstr Surg 2016;137:1690-9. [Crossref] [PubMed]
- Robinson EC, Antony AK. Five Questions About Direct-to-Implant Breast Reconstruction: What You Need to Know. Plastic Surgery Educational Network. Feb 2017. Available online: http://www.psenetwork.org/news-detail/five-questions-about-direct-to-implant-breast-reco
- Elswick SM, Harless CA, Bishop SN, et al. Prepectoral Implant-Based Breast Reconstruction with Postmastectomy Radiation Therapy. Plast Reconstr Surg 2018;142:1-12. [Crossref] [PubMed]


