Hybrid breast reconstruction—the best of both worlds
Introduction
Beyond merely achieving an adequate size breast mound, the goal of modern breast reconstruction is to restore a breast shape that is as close to “normal” as possible. If the purpose of reconstructive surgery is as Tagliacozzi said “to buoy up the spirit, and help the mind of the afflicted” then we must aim to achieve as natural a result as possible. Fortunately, advances in surgical techniques over the past four decades have pushed the boundaries of what is achievable in reconstructive surgery. Skin and nipple sparing mastectomy have decreased the severity of the mastectomy deformity (1,2). Meanwhile, perforator techniques have decreased the morbidity of autologous reconstruction (3). Moreover, the widespread use of autologous fat grafting allows for an enhanced aesthetic result in all reconstructions (4). These advances represent important tools that permit the reconstructive surgeon to better address patient wishes and desires.
However, despite these advances, there remain inherent limitations of established methods of breast reconstruction. For example, implant based reconstructions rely on the overlying soft tissue envelope to provide adequate cover to mask rippling and palpability of the implant. Variables that impact the soft tissue quality such as thin mastectomy skin flaps and radiotherapy can significantly impact the reconstructive outcome. Meanwhile, autologous techniques are fundamentally limited by the amount of available donor tissue, with flap design being determined by perforator location. For example, high peri-umbilical perforators are often associated with higher donor site scars than those patients undergoing aesthetic abdominoplasty. Additionally, bilateral reconstructions frequently lack both size and projection.
One solution to the limitations of pure implant or autologous techniques is to perform a combined approach such as the latissimus dorsi (LD) flap with an implant (5). This technique allows for control of both the soft tissue envelope as well as size and projection of the breast mound. Aesthetically, this method produces excellent results, however, the need for sacrifice of a major muscle represents a significant limitation particularly in those patients seeking bilateral reconstruction. Additionally, reconstruction of the natural ptosis of the breast is somewhat difficult to reliably achieve with LD flaps (6,7).
Starting in 2015, we began to implement a hybrid approach to breast reconstruction, where we combined implant placement with abdominal flap transfer in a single stage (8). The purpose of this review is to describe our experience with this novel method of reconstruction. Like LD flap and implant reconstruction this technique allows for control of both the soft tissue envelope as well as size and shape of the reconstruction. In contrast, however, donor-site morbidity is minimized. Because the implant size can vary according to the needs of the patient a large degree of flexibility is possible. Shape and projection can be maintained in thin patients and those needing bilateral reconstruction with relative ease. Furthermore, surgeons can limit the extent of donor site tissue harvest because the volume of the reconstruction does not come entirely from the abdominal donor site. Hence, we believe that hybrid breast reconstruction represents an important tool which can permit reconstructive surgeons to achieve an enhanced aesthetic outcome in select patients.
Surgical techniques
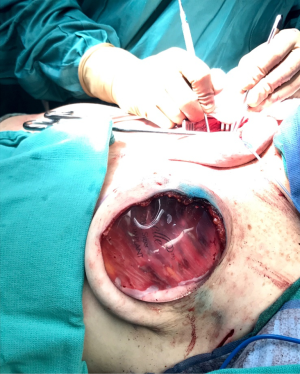
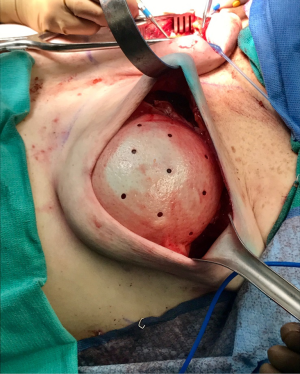
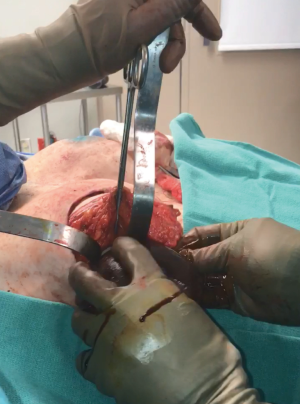
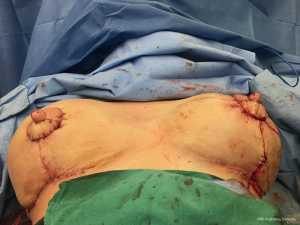
One of the principal concerns with placement of an implant at the time of free flap breast reconstruction is the potential for the implant to infringe on the pedicle resulting in a vascular embarrassment. This can be avoided with the use of a pre-pectoral acellular dermal matrix (ADM) “pocket” for the implant. A well-defined pocket to contain the implant fashioned from ADM is a hallmark of the hybrid autologous technique. The lower abdominal free flap [deep inferior epigastric perforator (DIEP) or muscle-sparing transverse rectus abdominis musculocutaneous (MS-TRAM)] is harvested using established techniques for flap harvest. Next, the internal mammary vessels are prepared with a dissection of the third rib cartilage and adjoining rib spaces. At this point a gel sizer is placed into the mastectomy defect (Figure 1). We utilize a sizer that is approximately 2–3 cm smaller than the base width of the patient’s breast. This allows the flap to be inset around the entire implant. After the sizer is placed a piece of ADM is sewn to the chest wall with interrupted 2-0 polyglactin sutures to completely cover the gel sizer (Figure 2). A 5-cm lateral portion of the ADM pocket is left unsewn and the sizer is removed from the ADM pocket through this lateral opening. Importantly, the sizer is positioned inferior to the internal mammary vessel harvest site and slightly lateral. At this point a perfectly sized and precisely positioned implant pocket has been created. The free flap anastomosis then proceeds in standard fashion and the flap is sutured to the mastectomy defect. Care is taken to loosely drape the pedicle over the ADM pocket. We ensure that there is enough redundancy in the pedicle to accommodate and anticipate 2–3 cm of additional excursion once an implant is placed. No attempt is made to shape the flap through suture techniques, it is simply sewn to the edges of the mastectomy defect. Once we are satisfied with the inset of the free flap, the definitive implant (identical in dimension to the sizer utilized) is then placed into the ADM pocket through the lateral opening (Figure 3). The flap pedicle and anastomosis are protected from any pressure exerted by the implant because of the precisely sized ADM pocket. Once we are satisfied with the position of the implant, the lateral ADM opening is closed with polyglactin sutures, thus, securing the implant. Lastly, the flap is re-draped over the implant/ADM and the lateral portion of the flap is secured to the chest wall.
Results
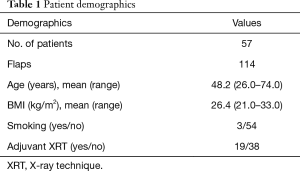
We have included all patients who have had the placement of an implant at the time of immediate autologous breast reconstruction over a 3-year period from January 2015 to January 2018 at both institutions after investigational review board approval. Patient characteristics are listed in Table 1. Importantly, the mean BMI of our patients undergoing the hybrid autologous approach was 26.4 kg/m2 (range, 21–33 kg/m2). This is slightly below the mean BMI for autologous patients at either institution, which is 28–29 kg/m2. While this is a relatively minimal difference it suggests the use of an implant in patients who may not have enough abdominal tissue volume for reconstruction of an adequately sized breast.
Full table
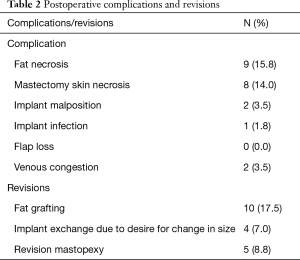
Table 2 displays the postoperative complication rate. Importantly, no flap loss was noted, thus, demonstrating the safety associated with this approach. There were, however, two incidences of venous compromise in this series. These two cases were related to an insufficient dissection of the internal mammary vessels. At the takeback procedure for both cases release of a tight band compromising the vein was all that was necessary and the implant was not removed. Both flaps were successfully salvaged with relatively minimal intervention. The rates of other complications were in line with our standard reconstruction practice. Importantly, there was a low rate of implant related complications of infection, malposition, or revisions for size.
Full table
Donor site aesthetics
A primary objective of the hybrid approach to breast reconstruction is to enhance the aesthetics of the donor site. One of the main features of autologous breast reconstruction with abdominal flaps that appeals to patients is the desire for an “abdominoplasty” result from the donor site harvest. DIEP flap breast reconstruction has been commonly marketed on the internet as a “tummy-tuck” breast reconstruction (9). However, there are some significant differences between abdominoplasty and autologous breast reconstruction. When an aesthetic surgeon is planning an abdominoplasty, the prime consideration is the degree of abdominal wall and skin laxity as well as the presence of rectus diastasis. The aesthetic surgeon has the luxury of focusing on donor site scar and can take away only the tissue that is in excess. However, the reconstructive surgeon planning free flap breast reconstruction must take into account perforator anatomy, which, however, often dictates a higher abdominal scar. Additionally, a major contributor to a high donor site scar is the need to take enough tissue to achieve an appropriate volume for breast reconstruction. This resection often provides an abdominal closure that is tight with a resulting scar that is routinely higher than in patients undergoing abdominoplasty.
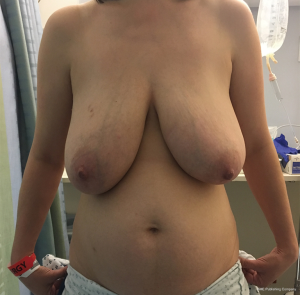
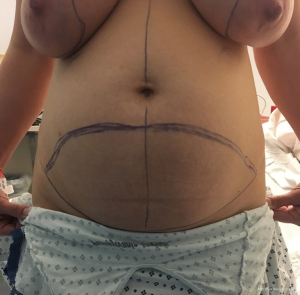
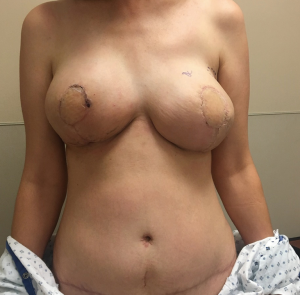
The ability to augment the free flap at the time of flap breast reconstruction with an implant has changed the planning of autologous breast reconstruction. Because an implant can supplement the needed volume, the reconstructive surgeon is not burdened with having to find all of the volume of the reconstruction from the abdominal tissue. Like abdominoplasty, only the tissue that is in excess needs to be removed. As long as there is sufficient skin laxity, the surgeon can use a larger implant if needed to make up for any flap volume deficiency. Additionally, simultaneous implant placement allows more patients to be candidates for autologous reconstruction. Thin patients without sufficient donor site volume would either not be deemed candidates or would require complex reconstructions involving multiple flaps to reconstruct a single breast (10,11). Figures 4-6 demonstrate the ability to harvest the entire flap from below the umbilicus, thus, resulting in a much lower and more aesthetically pleasing donor site. Given that the lowest portion of the abdominal flap has less fat, this shortage can be overcome by utilizing a larger implant.
Adjunct procedures
Another advantage of the hybrid approach is that revisionary procedures such as autologous fat grafting and nipple reconstruction are much more successful when compared to either implant or flap reconstruction alone. Autologous fat grafting has been a useful adjunct to both sub-pectoral or pre-pectoral implant breast reconstruction because of its ability to add additional volume and mask rippling and palpability of the implant. However, fat grafting in this setting is limited by the ability of the donor site to accept the fat graft. With the hybrid approach, fat grafting can be done in a staged manner into the core of a very well vascularized flap instead of the relatively thin skin envelope of an implant alone reconstruction. The thickness of the flap allows for multiple planes of smaller aliquots of fat to be deployed. This should lead to a greater volume of and better take of the grafts.
Furthermore, traditional nipple reconstruction in both autologous and implant based reconstructions is plagued by a decrease in projection over time (12). This is most pronounced in implant-based reconstructions. Nevertheless, even in autologous reconstructions where there is ample soft tissue it is often difficult to achieve projection of the nipple or areola which is a hallmark of the normal nipple-areolar complex. The presence of an implant underneath the free flap presents the ideal environment for nipple reconstruction because there is ample soft tissue, a thicker dermis, and a degree of pressure from the implant pushing the soft tissue forward (Figures 7,8).
Radiation [X-ray technique (XRT)]
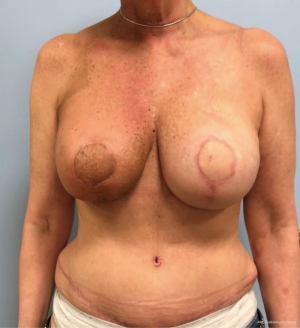
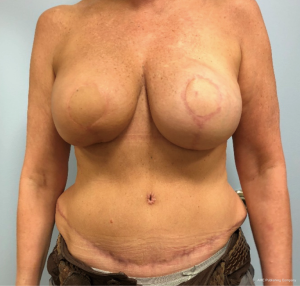
The thin patient who will need postoperative radiotherapy is one of the most challenging patients who presents to the plastic surgeon for breast reconstruction. Implant-based reconstructions in these patients have high rates of failure as well as aesthetic shortcomings (13,14). Pre-pectoral implant reconstruction has helped with this because much of the capsular contracture that was seen with sub-pectoral implant reconstruction was due to contracture and fibrosis of the muscle (15). Nonetheless, pre-pectoral reconstruction relies even more heavily on the quality of the overlying soft tissue. Because radiation worsens the overlying soft tissue envelope, many patients undergoing postoperative radiation will experience complications post-radiation.
Autologous reconstruction in the thin patient is also challenging. Namely, it is challenging to achieve a pleasing result with insufficient flap volume. While it is always possible to augment the reconstruction with an implant at a later timepoint, additional challenges exist in patients who have undergone radiotherapy. Hence, the aesthetic results are oftentimes not ideal. Once the mastectomy skin flap has healed to a smaller free flap it is hard to re-expand that pocket back to the original size because the free flap and mastectomy skin no longer move independent of one another. This phenomenon is made even more significant after radiotherapy as the outer skin envelope is densely adhered to the smaller flap. Secondary augmentation of the flap with an implant in this scenario often results in a larger breast with a less than ideal shape and contour.
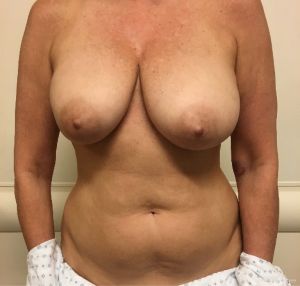
In contrast, performing a hybrid reconstruction in this patient population (Figures 9-11) can mitigate some of these challenges. The reconstruction is completed prior to XRT which means that the skin envelope is fully expanded to the desired size. This results in a relatively smooth contour to the reconstructed breast. Radiation then follows after the reconstruction. Given the thickness of the available soft tissue we have seen no problems with rippling or visibility of the implant and patients tolerate radiotherapy well. Similar to other forms of pre-pectoral implant reconstruction, we have not found capsular contracture to be a major concern in our 4-year experience.
Conclusions
Hybrid breast reconstruction is a useful addition to the range of reconstructive options available to meet our patients’ goals. This technique can avoid some of the limitations of traditional forms of breast reconstruction. The flap provides for a generous amount of soft tissue coverage over the implant so that rippling and implant palpability is diminished. Importantly, the presence of the implant serves as a structural foundation for the reconstruction. Hence, the implant contributes to both core volume as well as enhances projection. A similar effect is seen in patients who undergo augmentation mastopexy versus mastopexy alone. The impact of the implant is not only felt on the aesthetic outcome of the breast, but it also heavily impacts the donor site. Without having to find the entire reconstruction volume on the abdomen, the donor site scar can be lowered and the donor site will be left less tight as a result. This degree of flexibility adds to the creativity of the final outcome. In addition, we have found that adjunctive procedures such as fat grafting and nipple reconstruction are improved by the enhanced projection and improved soft tissue envelope provided by the hybrid approach. Lastly, the adverse impacts of radiation are better tolerated with the structure provided by the implant and the greater amount of soft tissue provided by the flap.
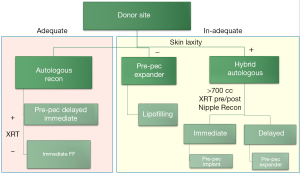
The advent of hybrid reconstruction has allowed us to think differently about breast reconstruction as a whole. We now utilize the algorithm displayed in Figure 12. The primary determinant of the type of reconstruction is the availability of the abdominal donor site. If the donor site is adequate to achieve the desired breast size and shape, traditional flap-based reconstruction is offered. However, if there is a paucity of abdominal tissue volume we examine the patient for adipofascial laxity. The criteria we utilize is if the patient would see an aesthetic benefit from abdominoplasty. If there is sufficient laxity in the absence of volume, the patient is a candidate for a hybrid approach. If there is insufficient laxity we would steer the patient towards pre-pectoral implant-based reconstruction.
Hybrid breast reconstruction provides a solution for many of the traditional limitations of implant and autologous reconstruction. This technique is a useful addition to our toolkit for reconstruction to help us meet the needs and expectations of our patients for superior outcomes in both the breast and abdomen.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Momeni is a consultant for Allergan, AxoGen, and Stryker. Dr. Kanchwala is a consultant for Allergan and AxoGen.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Tousimis E, Haslinger M. Overview of indications for nipple sparing mastectomy. Gland Surg 2018;7:288-300. [Crossref] [PubMed]
- Ashikari AY, Kelemen PR, Tastan B, et al. Nipple sparing mastectomy techniques: a literature review and an inframammary technique. Gland Surg 2018;7:273-87. [Crossref] [PubMed]
- Seidenstuecker K, van Waes C, Munder BI, et al. DIEAP flap for safe definitive autologous breast reconstruction. Breast 2016;26:59-66. [Crossref] [PubMed]
- Kanchwala SK, Glatt BS, Conant EF, et al. Autologous fat grafting to the reconstructed breast: the management of acquired contour deformities. Plast Reconstr Surg 2009;124:409-18. [Crossref] [PubMed]
- Taglialatela Scafati S, Cavaliere A, Aceto B, et al. Combining Autologous and Prosthetic Techniques: The Breast Reconstruction Scale Principle. Plast Reconstr Surg Glob Open 2017;5:e1602. [Crossref] [PubMed]
- Chu MW, Samra F, Kanchwala SK, et al. Treatment Options for Bilateral Autologous Breast Reconstruction in Patients with Inadequate Donor-Site Volume. J Reconstr Microsurg 2017;33:305-11. [Crossref] [PubMed]
- Momeni A, Kanchwala S. Hybrid Pre-pectoral Breast Reconstruction - A Surgical Approach that Combines the Benefits of Autologous and Implant-based Reconstruction. Plast Reconstr Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Momeni A, Kanchwala SK. Improved pocket control in immediate microsurgical breast reconstruction with simultaneous implant placement through the use of mesh. Microsurgery 2016. [Epub ahead of print]. [PubMed]
- Tang SYQ, Israel JS, Poore SO, et al. Facebook Facts: Breast Reconstruction Patient-Reported Outcomes Using Social Media. Plast Reconstr Surg 2018;141:1106-13. [Crossref] [PubMed]
- Rozen WM, Patel NG, Ramakrishnan VV. Increasing options in autologous microsurgical breast reconstruction: four free flaps for 'stacked' bilateral breast reconstruction. Gland Surg 2016;5:255-60. [PubMed]
- Mayo JL, Allen RJ, Sadeghi A. Four-flap Breast Reconstruction: Bilateral Stacked DIEP and PAP Flaps. Plast Reconstr Surg Glob Open 2015;3:e383. [Crossref] [PubMed]
- Momeni A, Becker A, Torio-Padron N, et al. Nipple reconstruction: evidence-based trials in the plastic surgical literature. Aesthetic Plast Surg 2008;32:18-20. [Crossref] [PubMed]
- Yun JH, Diaz R, Orman AG. Breast Reconstruction and Radiation Therapy. Cancer Control 2018;25:1073274818795489. [Crossref] [PubMed]
- Zhao R, Tran BNN, Doval AF, et al. A Multicenter Analysis Examining Patients Undergoing Conversion of Implant-based Breast Reconstruction to Abdominally based Free Tissue Transfer. J Reconstr Microsurg 2018;34:685-91. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]