
The role of fat grafting in prepectoral breast reconstruction
Introduction
Prosthetic breast reconstruction is the most common technique for post-mastectomy breast reconstruction (1). Timing may be either immediate or delayed, one- or two-stage. Moreover, the implant device itself may be placed above the muscle, completely under the muscle, or partially under the muscle. In the United States of America, most plastic surgeons tend to perform breast reconstruction in two stages (2).
The location of prosthetic devices continues to evolve as the techniques and technologies available to plastic surgeons continue to undergo advancement. Historically, breast reconstruction was performed with the implant in the subcutaneous plane (above the muscle), however lack of tissue support for the implant resulted in unacceptably high rates of flap necrosis and capsular contracture (3). These complications were partially mitigated by moving the implant into a total submuscular position (4). However, this position had the disadvantages of animation deformity, loss of muscular function, and pain (5,6). Introduction of acellular dermal matrices in the early 2000’s then allowed surgeons to place the implant only partially under the pectoralis muscle thereby reducing, but not eliminating, the morbidity associated with total submuscular coverage. However, acellular dermal matrix has subsequently also allowed the resurgence of prepectoral reconstruction, mitigating many of the difficulties that plagued early subcutaneous reconstruction (7). Recently, the rise of nipple sparing mastectomies has enhanced the aesthetic demand of breast reconstruction. This coupled with tissue perfusion assessment technologies that allow adequate assessment of flap viability, has resulted in a paradigm shift towards prepectoral breast reconstruction with the use of acellular dermal matrices.
Without submuscular or partial subpectoral placement of the device, there is a clear step-off between the chest wall and the prepectoral implant. The primary means for correcting these deformities is autologous fat grafting. For over a century, fat grafting has been a cornerstone in soft tissue reconstruction (8). During 2016, nearly 30% of all breast reconstruction cases utilized autologous fat grafts—a total of over thirty thousand patients (2). Despite their versatility and relative ease-of-use, fat grafts have been fraught with complications. Such issues include high resorption rates, oil cyst formation, and fat necrosis/calcification. Volume retention 140 days post-operatively stands around 25% to 50% for small and large volume grafts, respectively (9,10).
As the human body has a finite amount of graftable adipose tissue, repetitive extraction and grafting is neither desirable nor physically feasible, particularly for thin patients (11). Though developments have been made to improve the harvesting and preparation of grafted fat, little has been found to improve retention or reduce cyst formation (12). Thus, careful preoperative selection is required before pursuing prepectoral implantation and, for the prepectoral patient with rippling deformity, a strategic fat grafting approach.
Operative techniques
The first decision the operative surgeon must make is when to offer fat grafting to the prepectoral reconstructive patient. While it can be offered upon initial tissue expander placement, we advocate waiting until permanent implants are placed. Most importantly, it allows for definitive pathology confirming negative margins to return and the patient’s final treatment plan to be established. Additionally, once a patient is fully expanded to their satisfaction, mastectomy flap contour irregularities and areas of thin skin can become more pronounced, showing the surgeon and patient where volume augmentation may be most necessary. Finally, delaying fat grafting until permanent implant placement avoids further compromise to tissue flaps by additional volume distention.
Fat grafting during reconstruction with implants has a three-fold goal, filling in contour irregularities from mastectomy, augmenting areas of noticeable rippling, and augmenting certain areas to achieve a more ideal breast. As with the subpectoral population, the prepectoral reconstructive patient often benefits from multiple rounds of fat grafting, each addressing one or multiple goals. Prepectoral reconstructive patients should be counseled that achieving the optimal aesthetic outcome could require more than one procedure to achieve optimal results. In our experience, the benefits of prepectoral reconstruction are partnered with more rippling and more contour deformities. As a result, we typically see prepectoral patients needing more fat in the upper pole and areas of contour irregularity than individuals with a subpectoral reconstruction. However, it is imperative that the aesthetic goals of the patient always guide further procedures and subsequent harvests.
Contour irregularities occur frequently post-mastectomy and may be especially apparent when a patient is standing. It is essential that the operative surgeon mark a patient pre-operatively in the standing position, highlighting areas that would benefit from fat augmentation. These contour irregularities may be especially visible in a patient undergoing prepectoral reconstruction given the lack of muscle providing bulk to the flaps. A secondary goal should be to use additional fat harvested to augment certain areas of the breast, for example in the midline to build cleavage, adding upper pole fullness, or under the intended nipple position to provide additional projection. Reducing the appearance of implant rippling is the third goal of fat grafting (Figure 1). This is often addressed in secondary fat grafting procedures once the permanent implant has been placed.
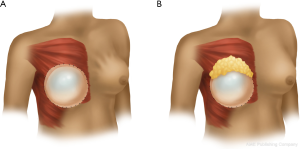
The location of lipoaspiration must also be selected and marked prior to entering the operating room. Typical harvest sites include the abdomen, flanks, and legs. While certain aspiration sites were initially thought to produce better graft take, these theories have not been supported by recent studies (13). In our favored technique, we select the minimum number of sites per fat grafting procedure to avoid reharvesting fat from a previous lipoaspiration site. Care must be taken not to create contour deformities. In addition, this needs to be discussed preoperatively with the patient for appropriate counseling.
At our institution, the preferred donor sites for fat grafting are the flanks, abdomens, and thighs based on the availability of subcutaneous adipose tissue and patient preference. Several studies evaluating the effect of donor site selection of fat graft retention and histologic parameters (14,15) have failed to show any association between the two. In the absence of new data associating donor site selection with surgical outcomes, decision should be made with patient safety and specific preferences in mind. Regardless of donor site, the risk for contour irregularities can be minimized by avoiding excess liposuction of the superficial layer of fat and staying clear of zones of adherence, including the lateral gluteal depression, gluteal crease, distal posterior thigh, mid-medial thigh, and inferolateral iliotibial tract (16).
In our preferred technique, our patients typically undergo their first fat grating at the same time as tissue expander exchange for permanent implant placement. At this time, mastectomy contour deformities, areas of thin skin, and medial and upper pole fullness can already be accurately analyzed. In secondary fat grafting procedures, we generally address areas of implant rippling, further contour irregularities, and finally the patient’s aesthetic concerns such as building more upper pole fullness.
Compared to subpectoral patients, prepectoral reconstructive patients typically require more fat injection into the upper pole and have more areas of contour deformities that require correction. We find rippling is also more apparent in the prepectoral patient population, a deformity that is generally addressed in secondary fat grafting procedures and requiring larger amounts of lipoaspirate to correct with satisfaction.
Infiltration of harvested fat is done with additional caution in the prepectoral population. The thin flap places the implant at risk of rupture by the cannula or bacterial seeding from skin flora. Care is taken to place the lipoaspirate in the subcutaneous plane, using a second hand to protect the underlying implant or vascular anastomosis in the event of autologous reconstruction. Given the known high rate of fat resorption, overcorrection of deformities can be tempting; however, we discourage this technique. The lack of subcutaneous tissue makes these flaps particularly sensitive to pressure necrosis.
In addition, the effect of fat processing techniques on cell populations and graft survival has been an active area of research over the past decade. Nonetheless, high quality, prospective clinical data is still lacking to guide surgical decision-making. Recent animal studies have suggested that processing fat on Telfa gauze may improve outcomes compared to centrifugation (17), however, this technique may not be feasible for large volumes of lipoaspirate and these findings have not been sufficiently replicated in human trials (13,18-20). At this time, our preferred technique is to use the Revolve (LifeCell Corp., Bridgewater, NJ, USA) system, which provides a combination of filtration of the lipoaspirate with serial washing and spinning. We find the system to be fast, easy-to-use, and reliable in our hands, and early pre-clinical data have demonstrated lower amounts of free oil and blood in Revolve specimens without any adverse effect on fat retention rates when compared to decanting and centrifugation (12).
Discussion
Although not entirely a new concept, prepectoral implant-based breast reconstruction has reemerged as a promising alternative to total or partial sub-muscular implant coverage following mastectomy (21-23). The advantages of this technique are manifold, and stem from its less invasive nature with decreased surgical and anesthesia times (24-27). By virtue of avoiding manipulation of the pectoralis major muscle, patients will likely experience lower levels of post-operative pain and muscle spasm (24,27). Furthermore, maintaining the muscle in its anatomical position obviates any concern for animation deformity (28) or loss of strength/function in active women (3).
Historically, the prepectoral approach was abandoned due to unacceptably high complication rates, including capsular contracture, flap necrosis, implant descent/migration, and the need for explantation (29). Even now, decreased soft tissue coverage in the pre-pectoral plane is less effective in disguising implant rippling or camouflaging the edges of the implant particularly in the upper pole of thin women (9). However, recent advances in both surgical technique and technology, including new generation expanders and implants, the use of acellular dermal matrices, intraoperative flap perfusion analysis, and fat grafting, have begun to address these concerns, allowing plastic surgeons to revisit this promising concept (24,30-36).
Of these advances, fat grafting in particular has established itself as an important adjunct to all types of breast reconstruction, especially those performed prepectorally (22,37). Although a number of techniques for fat grafting have been described (38), they all aim to augment the reconstructed breast, camouflaging irregularities in its shape and texture and more closely approximating a natural breast contour. Fat grafting also provides the added benefit of being minimally invasive and removing unwanted fat from potential donor areas. As many as 30% of all breast reconstructions now undergo fat grafting, and while complications including cyst formation, fat necrosis, or even infection are all possible, the procedure is generally well tolerated with high rates of patient satisfaction (38).
In prepectoral breast reconstruction, the pectoralis major muscle is not available to provide an additional layer of soft tissue coverage over the upper pole of the implant. These thinner flaps provide less fullness in the upper pole of the breast and do less to camouflage the edges of the implant or wrinkles in the outer shell that manifest themselves as skin rippling or contour irregularities. As such plastic surgeons have turned to autologous fat to reinforce the soft tissue and address the resultant deformities. Along the lower pole, ADM typically serve as an internal corset, supporting the weight of the implant but also blending implant surface irregularities. Although some surgeons extend the ADM coverage to encompass the entire tissue expander or implant, or at least the entire anterior surface (39,40), we prefer to use it to cover only the inferior pole of the tissue expander. This practice minimizes material costs while still affording structural support and decreasing the risk of capsular contracture (41).
Instead, we offer all patients autologous fat grafting at the time of tissue expander-implant exchange in order to address the soft tissue deficiency along the upper pole of the breast. The relatively thin flaps without muscle or ADM limit the volume of fat that can be transferred at one time, however the procedure can be successfully performed even when the subcutaneous tissue layer is found to be thin (30). For the majority of patients undergoing two-stage implant-based reconstruction a single round of fat grafting at the time of exchange is sufficient and no additional revisions are required; only 15–18% of patients can expect to require a second round down the line (22,30). Although rare in our practice, patients who desire direct to implant prepectoral breast reconstruction should be forewarned that they will likely require at least a second surgical procedure with autologous fat grafting. We do not advise fat grafting at the time mastectomy, as the grafts require a healthy vascular bed for survival and the instrumentation of the flaps may further the risk of necrosis and poor wound healing.
Several authors have reported technical considerations that, in addition to autologous fat grafting may reduce the incidence of rippling in the reconstructed breasts (21,24). In two-stage reconstructions, the tissue expander can be left under filled so that the larger implant is placed into a tight pocket without any redundant or loose skin. Furthermore, implant selection plays a significant role in prepectoral breast reconstruction outcomes. Implants must closely match the native breast width and be filled to almost near the outer shell’s capacity in order to minimize its rippling potential. Form stable gel implants may also serve to prevent rippling with their high cohesive gels (36). It is important to note, however, that even if rippling is avoided, most patients would still benefit from autologous fat grafting at the time of expander-implant exchange in order to augment the deficient upper pole and create a more natural “tear drop” shape to the breast.
Although a number of publications have begun to explore outcomes following pre-pectoral breast reconstruction, and specifically fat grafting, the literature is relatively still in its infancy and long-term outcomes are lacking (21,22,24,27,28,30,31,39,42). No study to date has evaluate complication rates within this cohort, and it remains to be seen if the thinner flaps result in difference in graft survival, cyst formation, or fat necrosis. Furthermore, the effect of autologous fat grafting on patient reported outcomes following prepectoral breast reconstruction remains to be seen. Nonetheless, preliminary evidence supports the benefits of prepectoral breast reconstruction (24,27,38), and when combined with autologous fat grafting, this technique has the potential to provide excellent aesthetic and functional patient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: JM Sacks serves as a Consultant/Speaker for Allergan. The other authors have no conflicts of interest to declare.
References
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: Increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [Crossref] [PubMed]
- American Society of Plastic Surgeons 2016. Plastic Surgery Statistics Report 2016.
- Highton L, Johnson R, Kirwan C, et al. Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1488. [Crossref] [PubMed]
- Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: A comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312-7. [Crossref] [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- de Haan A, Toor A, Hage JJ, et al. Function of the pectoralis major muscle after combined skin-sparing mastectomy and immediate reconstruction by subpectoral implantation of a prosthesis. Ann Plast Surg 2007;59:605-10. [Crossref] [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010;126:1842-7. [Crossref] [PubMed]
- Zielins ER, Brett EA, Longaker MT, et al. Autologous Fat Grafting: The Science Behind the Surgery. Aesthet Surg J 2016;36:488-96. [Crossref] [PubMed]
- Sommer B, Sattler G. Current Concepts of Fat Graft Survival: Histology of Aspirated Adipose Tissue and Review of the Literature. Dermatol Surg 2000;26:1159-66. [Crossref] [PubMed]
- Choi M, Small K, Levovitz C, et al. The Volumetric Analysis of Fat Graft Survival in Breast Reconstruction. Plast Reconstr Surg 2013;131:185-91. [Crossref] [PubMed]
- Weichman KE, Broer PN, Thanik VD, et al. Patient-Reported Satisfaction and Quality of Life following Breast Reconstruction in Thin Patients: A Comparison between Microsurgical and Prosthetic Implant Recipients. Plast Reconstr Surg 2015;136:213-20. [Crossref] [PubMed]
- Ansorge H, Garza JR, McCormack MC, et al. Autologous fat processing via the Revolve system: quality and quantity of fat retention evaluated in an animal model. Aesthet Surg J 2014;34:438-47. [Crossref] [PubMed]
- Strong AL, Cederna PS, Rubin JP, et al. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast Reconstr Surg 2015;136:897-912. [Crossref] [PubMed]
- Li K, Gao J, Zhang Z, et al. Selection of donor site for fat grafting and cell isolation. Aesthetic Plast Surg 2013;37:153-8. [Crossref] [PubMed]
- Varghese J, Griffin M, Mosahebi A, et al. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther 2017;8:45. [Crossref] [PubMed]
- Rohrich RJ, Smith PD, Marcantonio DR, et al. The zones of adherence: role in minimizing and preventing contour deformities in liposuction. Plast Reconstr Surg 2001;107:1562-9. [Crossref] [PubMed]
- Canizares O, Thomson JE, Allen RJ, et al. The Effect of Processing Technique on Fat Graft Survival. Plast Reconstr Surg 2017;140:933-43. [Crossref] [PubMed]
- Tuin AJ, Domerchie PN, Schepers RH, et al. What is the current optimal fat grafting processing technique? A systematic review. J Craniomaxillofac Surg 2016;44:45-55. [Crossref] [PubMed]
- Fisher C, Grahovac TL, Schafer ME, et al. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg 2013;132:351-61. [Crossref] [PubMed]
- Cleveland EC, Albano NJ, Hazen A. Roll, Spin, Wash, or Filter? Processing of Lipoaspirate for Autologous Fat Grafting: An Updated, Evidence-Based Review of the Literature. Plast Reconstr Surg 2015;136:706-13. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: A safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565-7. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Kim JYS, Khavanin N, Rambachan A, et al. Surgical duration and risk of venous thromboembolism. JAMA Surg 2015;150:110-7. [Crossref] [PubMed]
- Mlodinow AS, Khavanin N, Ver Halen JP, et al. Increased anaesthesia duration increases venous thromboembolism risk in plastic surgery: A 6-year analysis of over 19,000 cases using the NSQIP dataset. J Plast Surg Hand Surg 2015;49:191-7. [Crossref] [PubMed]
- Ter Louw RP, Nahabedian MY. Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:51S-9S. [Crossref] [PubMed]
- Jones G, Yoo A, King V, et al. Prepectoral Immediate Direct-to-Implant Breast Reconstruction with Anterior AlloDerm Coverage. Plast Reconstr Surg 2017;140:31S-8S. [Crossref] [PubMed]
- Nahabedian MY, Glasberg SB, Maxwell GP. Introduction to “Prepectoral Breast Reconstruction”. Plast Reconstr Surg 2017;140:4S-5S. [Crossref] [PubMed]
- Elswick SM, Harless CA, Bishop SN, et al. Prepectoral Implant-Based Breast Reconstruction with Postmastectomy Radiation Therapy. Plast Reconstr Surg 2018;142:1-12. [Crossref] [PubMed]
- Khavanin N, Gust MJ, Grant DW, et al. Tabbed tissue expanders improve breast symmetry scores in breast reconstruction. Arch Plast Surg 2014;41:57-62. [Crossref] [PubMed]
- Jordan SW, Khavanin N, Fine NA, et al. An algorithmic approach for selective acellular dermal matrix use in immediate two-stage breast reconstruction: Indications and outcomes. Plast Reconstr Surg 2014;134:178-88. [Crossref] [PubMed]
- Jordan SW, Khavanin N, Kim JYS. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg 2016;137:1104-16. [Crossref] [PubMed]
- Khavanin N, Clemens MW, Pusic AL, et al. Shaped versus round implants in breast reconstruction: A multi-institutional comparison of surgical and patient-reported outcomes. Plast Reconstr Surg 2017;139:1063-70. [Crossref] [PubMed]
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: Results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Delay E, Guerid S. The Role of Fat Grafting in Breast Reconstruction. Clin Plast Surg 2015;42:315-23. [Crossref] [PubMed]
- Spear SL, Coles CN, Leung BK, et al. The Safety, Effectiveness, and Efficiency of Autologous Fat Grafting in Breast Surgery. Plast Reconstr Surg Glob Open 2016;4:e827. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: A new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Downs RK, Hedges K. An Alternative Technique for Immediate Direct-to-Implant Breast Reconstruction-A Case Series. Plast Reconstr Surg Glob Open 2016;4:e821. [Crossref] [PubMed]
- Kim I-K, Park SO, Chang H, et al. Inhibition Mechanism of Acellular Dermal Matrix on Capsule Formation in Expander-Implant Breast Reconstruction After Postmastectomy Radiotherapy. Ann Surg Oncol 2018;25:2279-87. [Crossref] [PubMed]
- Hudson DA. Optimizing Aesthetics with Prosthetic Prepectoral Breast Reconstruction. Plast Reconstr Surg 2018;141:611e-2e. [Crossref] [PubMed]


