The bioengineered prosthetic breast reconstruction: advancements, evidence, and outcomes
Introduction
2017 statistics complied by the American Society of Plastic Surgeons have demonstrated that of the 106,295 breast reconstructions performed, 86,979 (81.2%) were performed with prosthetic devices (1). The reasons for this include patient preference, improved mastectomy and reconstructive techniques, and improved outcomes (2). This paradigm shift is multifactorial and related to lifestyle choices, less invasive operations, a more rapid recovery, the ability to deliver excellent aesthetic outcomes.
It can be stated that reconstructive breast surgery is now an aesthetic operation. Patients and surgeons no longer find it acceptable to reconstruct just a breast mound. Current expectations are to reconstruct a breast that is naturally contoured and positioned and that provides optimal symmetry. Factors that have contributed to this paradigm shift include the use of acellular dermal matrix (ADM), nipple sparing mastectomy (NSM), fat grafting, better breast implants, and prepectoral reconstruction. This manuscript will review these factors in greater detail and discuss the evidence supporting these innovations.
NSM
Over the past decade, the rate of NSM for therapeutic indications has increased. In a recent review of 114,819 patients from the National Cancer Data Base, the incidence of NSM had increased from 2.9% to 8% between 2010 and 2013 (3). Traditional indications for NSM included tumors that were greater than 3 cm from the nipple areolar complex (NAC), less than 5 cm in size, and no angiolymphatic invasion (4,5). Current strategies have evolved such that margin status around the tumor is the primary determinant rather than distance from the NAC or tumor dimensions. Systematic reviews focused on NSM have demonstrated that complications occur in 11.2–22.3%, necrosis of the NAC occurs in 2.9–7%, and that local regional recurrence occurs in 1.8–2.3% of patients (5-7) (Table 1).

Full table
The oncologic aspects of NSM continue to be discussed and studied. In a recent systematic review of 29 studies, several observations with statistical significance were made (5). With regard to tumor size, the overall incidence of NAC involvement was 9.8% when the tumor was <2 cm, 13.3% for tumors ranging from 2–5 cm, and 31.8% for tumors >5 cm (P<0.05). With regard to tumor location, the incidence of NAC involvement was 35.2% for central or retroareolar tumors and 9.7% for peripheral tumors (P<0.05). Multicentric tumors involved the NAC in 29.6% of cases, whereas solitary tumors had a 12.4% involvement (P<0.05). The incidence of NAC involvement in patients with a positive lymph node was 24.4%, whereas it was 10% in patients without lymph node involvement (P<0.05). Lymphovascular invasion resulted in NAC involvement in 35.6% of patients whereas it was only 12.4% without lymphovascular invasion (P<0.05). With regard to tumor type, the incidence of nipple involvement was 14.9% for invasive ductal carcinoma, 15.3% for ductal carcinoma in situ (DCIS), 17.2% for invasive lobular carcinoma, and 17.2% for invasive ductal carcinoma.
The technical aspects of NSM continue to evolve as breast and plastic surgeons strive to improve aesthetic outcomes while prioritizing oncologic safety (7,8). Perhaps the most important determinant of a good outcome following prosthetic breast reconstruction is the presence of well-perfused mastectomy skin flaps. When these flaps are too thin or widely undermined vascularity is usually compromised and the likelihood of skin necrosis, reconstructive failure and a poor outcome is increased (9). It is important for breast surgeons and plastic surgeons to communicate and ensure the delivery of an optimized mastectomy skin flap with the goal to preserve the subcutaneous layer and vascularity without compromising oncologic integrity.
The incisions for NSM include radial, lateral, periareolar and inframammary (7). The choice of incision is based on several factors including degree of ptosis, type of reconstruction, breast volume, as well as patient and surgeon preference (Figure 1). In a systematic review, rates of partial or total NAC necrosis were evaluated and demonstrated a necrosis rate of 8.83% for radial, 17.8% for periareolar and 9.09% for inframammary incisions (7). Transareolar incisions demonstrated the highest rate of delayed healing at 81.8% (6). Incisional approach had no effect on local regional recurrence rates.
In women with moderate mammary hypertrophy or with breast ptosis that desire NSM, various approaches have been described. Spear has described a staged approach whereby a reduction mammaplasty or mastopexy is performed as a first stage followed by a second stage NSM (10). In a woman with breast cancer, the first stage is essentially an oncoplastic operation whereby a partial mastectomy is performed followed by reduction or mastopexy. The timing for the second stage depends upon whether the procedure was for oncologic or prophylactic indications. If oncologic, it is recommended that the NSM be performed approximately 1 month later to avoid delays in treatment. If prophylactic, the NSM should occur 3 months or later. An alternative approach to reduction mammaplasty is the nipple delay procedure. This involves creating a vertical incision below the NAC followed by undermining of the NAC with disruption of the pectoral/intercostal perforators (11,12). This enables the peripheral vascularity to become the dominant blood supply to the NAC. The NSM is usually performed 2–3 weeks later.
ADM
The use of ADM is arguably one of the main advancements contributing to the rise in prosthetic breast reconstruction. Benefits include soft tissue support, elasticity, less scar, and device compartmentalization (13). Its ability to incorporate into the adjacent soft tissue by fibroblast infiltration and revascularization is the foundation for its success (Figure 2). Tissue incorporation is facilitated using human skin donors, creating fenestrations or perforations in the ADM that serve as zones of adherence, and achieving a hand-in-glove fit into the mastectomy space. ADM can be used for direct-to implant as well as 2-stage reconstruction. It can be used for prepectoral as well as partial subpectoral placement of devices. The role of ADM following partial subpectoral device placement is to stabilize the position of the pectoralis major muscle (Figure 3). Failure to do often results in window-shading that is characterized by visibility of the inferior edge of the muscle during contraction. The role of ADM following prepectoral device placement is to provide tissue support and to compartmentalize the device on the chest wall (Figure 4). In both cases, ADM has the potential to minimize adverse inflammation and reduce the incidence of capsular contracture (14).
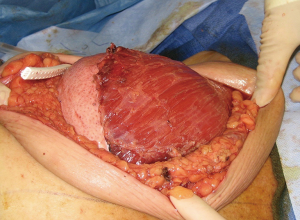
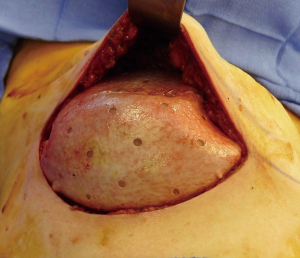
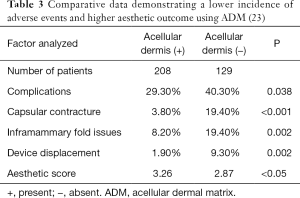
Perhaps one of the greatest benefits of ADM use is its ability to minimize scar formation around the implant thereby reducing the incidence of capsular contracture (14-19). Evidence for this comes from a variety of experimental and clinical studies focused on ADM performance (Table 2). Experimental studies in rabbits and primates comparing rates of capsule formation device implantation with and without ADM have demonstrated a lack of capsule formation in the ADM cohorts in contrast to thick capsule formation in the setting of no ADM (20,21). Histology of ADM following clinical use has demonstrated mild inflammation, collagen production, granulation and vascular proliferation (22). Native capsule on the other hand demonstrates abundant granulation, mild vascular proliferation, a moderate increase in collagen and inflammatory infiltrates (20). In another clinical study comparing outcomes in patients following prosthetic reconstruction with and without ADM, the incidence of capsular contracture was 3.80% in the setting of ADM and 19.40% without ADM (23) (Table 3). The association between inflammation and fibrosis in the setting of prosthetic devices is well known. Women with silicone gel implants that have capsular contracture are characterized with capsules with vascular proliferation as well as lymphocytic and mononuclear infiltrates (24).
Full table
The performance of ADM in the setting of radiation therapy has also been a topic of discussion. ADM has been demonstrated to revascularize and incorporate into the adjacent soft tissues in the setting of both pre and postoperative radiation, albeit at a slower rate (25). Experimental studies have demonstrated that capsules surrounding implants without ADM in the setting of radiation to be associated with prominent inflammatory infiltrates and pseudo-epithelial cells leading to prominent capsules whereas implants with ADM tend to have less cellular invasion and delayed or diminished pseudo-epithelial formation (26). ADM histology with and without radiation demonstrated no change in the relative proportion of cellularity, collagen content, elastin content, alpha smooth muscle actin and macrophage levels (27).
Clinical studies have demonstrated that infection rates following prosthetic reconstruction tend to be increased in the setting of radiation therapy but do not seem to vary based on the use of ADM (28). Studies have also examined the timing of radiation relative to prosthetic reconstruction with ADM. In most studies, complications are increased when radiation therapy precedes prosthetic reconstruction (28). The adverse effects of radiation therapy are unlikely to be altered by ADM (27). The effects of radiation are likely to be very similar when directed toward normal dermis or revascularized ADM.
Autologous fat grafting
The use of autologous fat grafting in the setting of prosthetic breast reconstruction has provided a valuable adjunct for plastic surgeons to correct contour and volume irregularities; however, it is not without controversy (29). Fat is metabolically active and consists of a diverse secretory cell population that includes cytokines, hormones, and growth factors (30-32). There are experimental studies suggesting that grafted fat may promote or accelerate cancer growth (33,34). In one such study, it was demonstrated that there was an increased potential for malignant transformation of progenitor cells in normal breast tissue in the presence of stromal vascular fraction (SVF) (33). In a similar study, it was found that human breast cancer cell viability increased from 45.5% to 95.5% in presence of adipose-derived mesenchymal stromal cells in vitro (34). Fortunately, clinical studies have not demonstrated malignant transformation or an increased in cancer recurrence in patients receiving autologous fat grafting (35,36). In one study, the biopsy rate after fat grafting was 7.4% without evidence of locoregional cancer recurrence (35). In another study, fat grafting after breast reconstruction did not adversely affect local tumor recurrence or survival on long-term follow-up (36). Although mammographic confusion following fat grafting is occasionally an issue, calcifications associated with fat grafting are characterized differently than calcifications associated with malignancy (37,38).
In light of these issues the FDA has partnered with the American Society of Plastic Surgeons to maintain a proper use protocol that includes minimal manipulation of the harvested fat during the processing phase as well as injecting fat where fat normally resides. Minimal manipulation includes the avoiding strategies such as enzymatic processing of the fat to increase the stem cell population known as the SVF. Because of studies such as these, the FDA considers SVR a drug that does not fall into the category of tissue. Further studies evaluating the safety and efficacy of autologous fat grafting to the breast are in progress.
Based on the safety profile of grafted fat, this procedure is commonly offered to patients following mastectomy and reconstruction. Fat grafting is most commonly used for the correction of contour deformities, implant rippling, volume discrepancies and to improve the quality of radiated skin. The latter benefit is presumed related to the positive effect of stem cells in the lipoaspirate (39). Complications of autologous fat grafting include oil cysts formation, resorption, fat necrosis, microcalcifications, infection, nodularity, and contour abnormalities (30,40).
Techniques for fat aspiration, harvest, filtration, and transfer have varied amongst surgeons but all have demonstrated success in the majority of patients (Figure 5). The technique of fat grafting involves the aspiration of fat usually from the abdomen or thigh, removal of the excess fluids, oils and blood remnants, followed by fat injection in the desired area. In some patients, percutaneous aponeurectomy is necessary to disrupt the fibrous connections within the subcutaneous tissues, especially following radiation therapy (41). In some patients, especially those that have received prior radiation, several sessions of fat grafting may be required (39). Because the fat is transferred without a blood supply, revascularization is acquired from the recipient site resulting in retention; however, when revascularization is not achieved, resorption of fat will occur. Fat retention following lipofilling in the setting of mastectomy or reconstruction has been demonstrated to range from 40–60% and dependent upon previous radiation, prosthetic or autologous, and injection volume (42).
The concept of total breast reconstruction with fat grafting alone has been explored (43). External expansion system applied over the mastectomy site has been used to stretch the skin and improve the vascularity. The device is utilized for defined period of time to expand the space in which the fat will be injected and to improve the vascularity. The expansion and added vascularity creates an ideal environment for grafted fat to acquire vascularity and survive. This can be supplemented with an implant if needed.
Prosthetic devices
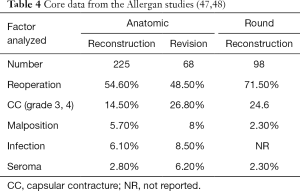
Prosthetic devices for breast reconstruction were first introduced in 1962 and have undergone significant modifications over the past 6 decades (44-46). Current devices can be filled with saline or silicone gel, have smooth or textured surfaces, and have a round or anatomic shape. The core studies have demonstrated that these devices are safe and effective with follow-up that ranges from 6–10 years (47-52). In all series, the reoperation rates tend to be higher than expected; however, reoperation does not imply device failure. In many cases, reoperation is performed electively and for aesthetic enhancement. Tables 4-6 review the core study data from the three manufacturers of breast implants available in the USA.
Prosthetic devices for reconstruction can be temporary, as in the case of tissue expanders, or permanent. Prosthetic breast reconstruction can be performed in 1 or 2 stages. Regardless of the stages, proper device selection is critical and optimized with biodimensional planning such that the device will closely match the footprint of the breast or mastectomy pocket. Two-stage reconstruction is performed using a tissue expander that is inserted into the post mastectomy space and filled with saline or air through an integrated port. Most tissues expanders are constant in terms of design and include a contoured shape, textured surface, suture tabs, and an integrated port. The suture tabs are to minimize the risk of device rotation of malposition (53). A recently introduced tissue expander that is remote-controlled, needle-free, and carbon dioxide-based that is now available and has been approved by the FDA (54). Tissue expanders are partially filled in the operating room and gradually filled over time to stretch the surrounding tissues with the goal of creating a natural breast mound. As with traditional expanders, these are removed and exchanged for a permanent implant following desired expansion.
There are a myriad of permanent implants available for prosthetic breast reconstruction. They vary in terms of size (100–800 cc), filler material (saline or silicone gel), surface (smooth or textured), and shape (round or anatomic). Current silicone gel devices are made with highly cohesive silicone gel that often results in less rippling and wrinkling. Shaped breast implants are able to maintain the natural contour profile of the device and result in a natural slope of the upper pole with less rippling and wrinkling. Current shaped implants have a textured surface that is presumed to provide better adherence to the surrounding soft tissue and to minimize implant rotation (55). There is clinical evidence demonstrating less capsular contracture with the use of textured surface devices (56). Shaped implants tend to increase projection along the lower pole of the breast and provide a gradual slope of the upper pole creating a natural breast shape. In general, shaped implants are useful when maximal control of the breast shape is necessary such as in patients with upper pole deficiency or a long torso (57). Round implants are available with smooth or textured surfaces and are sometimes preferred because they are softer than shaped devices and tend to move more like a natural breast.
When comparing silicone gel to saline implants, the majority of plastic surgeons and patients prefer silicone because they are soft and more closely resembles the natural breast. Some women however may not be comfortable with silicone gel breast implants based on reports from the early 1990s suggesting that they were associated with a myriad of problems such as chronic fatigue, connective tissue disorders, and altered immunity. Based on scientific evidence and numerous clinical studies, silicone gel breast implants have been demonstrated to be safe and effective by the FDA as well as the Institute of Medicine and not associated with the development of any of these disorders (44).
Breast implant associated-anaplastic large cell lymphoma (BIA-ALCL) has recently emerged as a public concern and is associated with the use of textured surface breast implants. BIA-ALCL is extremely rare with a reported incidence that ranges from 1:1,000–30,000 patients (58-60). It typically manifests as a late swelling of the reconstructed breast do to a fluid collection or seroma with a predilection for textured surface devices. There is growing evidence that this may arise from a bacterial strain, Ralstonia, that tends to reside more commonly on the surface of macro-textured devices causing chronic inflammation and malignant transformation (59,60). The average time to onset is approximately 8 years. Diagnosis is via serology demonstrating anaplastic lymphoma kinase (ALK) negative and CD-30 positive. Treatment includes total capsulectomy and device removal.
Despite the safety and efficacy of breast implants, they do not last forever with an average life span of 10–15 years. Over time, device failure will become more likely due to rupture and/or capsular contracture that usually requires removal or replacement. It is recommended that women with silicone gel breast implants have MRI every 3–5 years to assess implant integrity and rule out silent rupture.
Prepectoral breast reconstruction
The innovations and advancements with prosthetic breast reconstruction are many and include the use of ADM, fat grafting, NSM, and better implants. These advancements have enabled surgeons to now place breast implants in the prepectoral space rather than under the pectoralis major muscle (61-64). Subcutaneous reconstruction was initially performed in the 1970’s but abandoned because of the high complication rates that included skin necrosis (13.5%), device extrusion (6.7%), capsular contracture (56%), and explantation (28%) (65). Based on the initial failure of subcutaneous reconstruction, partial and total muscle coverage techniques became the standard. Despite the benefits of subpectoral device placement, shortcomings such as animation deformity with muscle contraction, pectoralis muscle spasm, and a generalized discomfort were common. The evolution to prepectoral placement was initiated with the abandonment of the Halstedian mastectomy principles that included aggressive mastectomy, thin flaps and wide undermining. Thus, the concept of the Bio-Engineered Breast has evolved as initially described by Dr. Maxwell based on these advancements that include nipple and skin sparing mastectomy, ADMs, autologous fat grafting, and improved prosthetic devices (61). The ability to place implants in the prepectoral space would not be possible if not for these innovations and advancements.
The benefits of pre-pectoral breast reconstruction are becoming well understood and can be explained based on anatomic and technical considerations (62-64). The pectoralis major muscle is no longer elevated or manipulated and as a consequence does not contribute to the pain, spasm and animation that was associated with partial subpectoral device placement. There is potential to reduce surgical and anesthesia time due to the simplicity of the technique. Most surgeons performing prepectoral reconstruction do so with the use of ADM to provide soft support. An addition benefit of ADM is to maintain low rates of capsular contracture. Capsular contracture rates using ADM tend to be less than capsular contracture rates without ADM (14,22). Long-term outcomes of prepectoral breast reconstruction are still lacking because the procedure is relatively new; however, outcomes with 2–3 years follow-up are encouraging (63,64,66,67). The principle limitation of prepectoral device placement is that adequate soft tissue support may be lacking in some cases; thus, proper patient selection is critical to minimize the risks of rippling, wrinkling and delayed healing. When adequate soft tissue support is lacking, delayed reconstruction is considered.
Prepectoral reconstruction in the setting of radiation therapy has become a major topic of discussion (68). When radiation therapy is delivered in the setting of a subpectoral or partial subpectoral device, it is common to observe skin tightening and elevation of the inframammary fold ranging from 1–4 cm. When radiation is delivered to devices in the prepectoral position, elevation of the inframammary fold is not observed or minimal. Theories explaining this observation suggest that the effects of radiation are more pronounced towards the pectoralis major muscle, especially when the inferior origin has been divided. The effect is manifested by contraction and foreshortening of the fibers of the pectoralis major resulting in the cephalad displacement of the prosthetic device.
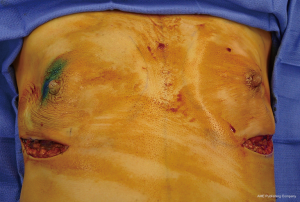
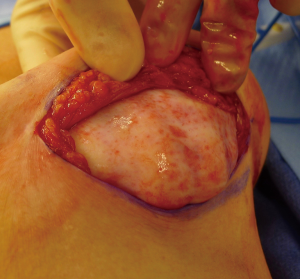
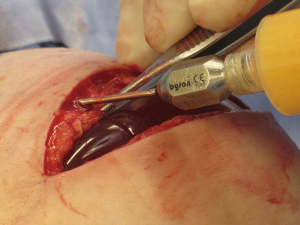
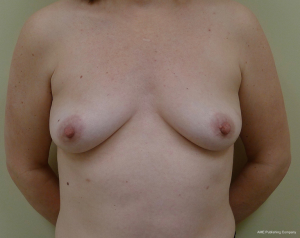
The technique of prepectoral reconstruction is simple. Mastectomy skin flaps are assessed for thickness and perfusion. Direct to implant as well as tissue expander—implant can be performed and based on patient desire and surgeon comfort (69,70). Prepectoral reconstruction can be performed with or without ADM; however, ADM use is more common (71,72). ADM assembly can be performed using on or off label techniques according to FDA guidelines. Because ADM is indicated for soft tissue support, the on-label techniques are based on placing the ADM into the breast pocket first followed by the device second. With the off-label technique, the ADM is wrapped around the tissue expander or implant before insertion into the mastectomy defect. The ADM assembly can be designed as a 360° wrap or 180° around the device. Figures 6-8 illustrate a patient following prepectoral reconstruction performed in two stages.
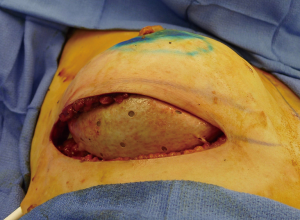
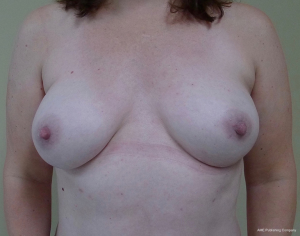
Recent clinical studies have supported the concept of prepectoral reconstruction and demonstrated the technique to be safe and effective. In a recent prospective multicenter study with data collected from 2014–2015 on 100 pre-pectoral breast reconstructions using Braxon dermal matrix, excellent outcomes were noted that included nipple necrosis or delayed healing occurred in 2% of patients at a mean follow-up of 17.9 months (73,74). In a retrospective review of 353 prepectoral reconstructions using ADM in 207 patients, Sigalove et al. demonstrated low rates of infection (4%), seroma (2%), and skin flap necrosis rate (2.5%) (63). In a retrospective review of 135 prepectoral reconstructions using ADM, Woo et al. demonstrated successful reconstruction in 96% of patients with minor complications occurring in 14% (64). Studies comparing outcomes between prepectoral and total muscle coverage techniques have demonstrated similar morbidities with regard to infection, superficial skin necrosis, and seroma (75) (Table 7). In addition, studies have confirmed that capsular contracture rates are lower when prepectoral reconstruction is performed with ADM (0%) compared to without ADM (12%) (76).
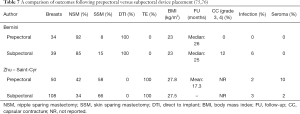
Full table
Summary
Prosthetic reconstruction has evolved and improved over the years based on the various innovations and advancements discussed. The use of ADM, autologous fat, improved mastectomy techniques, and improved devices remain the cornerstone of the bioengineered breast. The prepectoral concept represents the most recent advancement and may result in a paradigm shift with prosthetic breast reconstruction.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Nahabedian is a consultant for Allergan and Chief Surgical Office for PolarityTE.
Informed Consent: Informed consent has been obtained from the patients for publication of their figures.
References
- Available online:, ASPS statistics. Accessed June 23, 2018.www.plasticsurgery.org
- Albornoz CR, Bach PB, Mehrara BJ, et al. A Paradigm Shift in U.S. Breast Reconstruction: Increasing Implant Rates. Plast Reconstr Surg 2013;131:15-23. [Crossref] [PubMed]
- Sisco M, Kyrillos AM, Lapin BR, Wang CE, Yao KA. Trends and variation in the use of nipple-sparing mastectomy for breast cancer in the United States. Breast Cancer Res Treat 2016;160:111-20. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013;131:969-84. [Crossref] [PubMed]
- Headon HL, Kasem A, Mokbel K. Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch Plast Surg 2016;43:328-38. [Crossref] [PubMed]
- Endara M, Verma K, Chen D, et al. Breast reconstruction following nipple spring mastectomy:A systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- Dull B, Conant L, Myckatyn T, et al. Nipple-sparing mastectomies: Clinical outcomes from a single academic institution. Mol Clin Oncol 2017;6:737-42. [Crossref] [PubMed]
- Patel KM, Hill LM, Gatti ME, et al. Management of Massive Mastectomy Skin Flap Necrosis Following Autologous Breast Reconstruction. Ann Plast Surg 2012;69:139-44. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [Crossref] [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical Delay of the Nipple-Areolar Complex: A Powerful Technique to Maximize Nipple Viability Following Nipple-Sparing Mastectomy. Ann Surg Oncol 2012;19:3171-6. [Crossref] [PubMed]
- Nahabedian MY. Acellular Dermal Matrices in Primary Breast Reconstruction:Principles, Concepts, and Indications. Plast Reconstr Surg 2012;130:44S-53S. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 2007;59:250-5. [Crossref] [PubMed]
- Zienowicz RJ. Implant based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [Crossref] [PubMed]
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [Crossref] [PubMed]
- Namnoum JD. Expander/Implant Reconstruction with AlloDerm: Recent Experience. Plast Reconstr Surg 2009;124:387-94. [Crossref] [PubMed]
- Uzunismail A, Duman A, Perk C, et al. The effects of acellular dermal allograft (AlloDerm) interface on silicone-related capsule formation: an experimental study. Eur J Plast Surg 2008;31:179-85. [Crossref]
- Stump A, Holton LH 3rd, Connor J, et al. The Use of Acellular Dermal Matrix to Prevent Capsule Formation around Implants in a Primate Model. Plast Reconstr Surg 2009;124:82-91. [Crossref] [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular Cadaveric Dermis Decreases the Inflammatory Response in Capsule Formation in Reconstructive Breast Surgery. Plast Reconstr Surg 2010;126:1842-7. [Crossref] [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of Implant-Based Immediate Breast Reconstruction with and without Acellular Dermal Matrix. Plast Reconstr Surg 2011;128:403e-10e. [Crossref] [PubMed]
- Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and Morphological Conditions in Capsular Contracture Formed around Silicone Breast Implants. Plast Reconstr Surg 2007;120:275-84. [Crossref] [PubMed]
- Ibrahim HZ, Kwiatkowski TJ, Montone KT, et al. Effects of external beam radiation on the allograft dermal implant. Otolaryngol Head Neck Surg 2000;122:189-94. [Crossref] [PubMed]
- Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg 2009;123:807-16. [Crossref] [PubMed]
- Moyer HR, Pinell-White X, Losken A. The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction:clinical outcomes and histologic analysis. Plast Reconstr Surg 2014;133:214-21. [Crossref] [PubMed]
- Nahabedian MY. AlloDerm Performance in the setting of Breast Implants, Infection, and Radiation. Plast Reconstr Surg 2009;124:1735-40. [Crossref]
- Kling RE, Mehrara BJ, Pusic AL, et al. Trends in autologous fat grafting to the breast: a national survey of the American society of plastic surgeons. Plast Reconstr Surg 2013;132:35-46. [Crossref] [PubMed]
- Largo RD, Tchang LA, Mele V, et al. Efficacy, safety and complications of autologous fat grafting to healthy breast tissue: A systematic review. J Plast Reconstr Aesthet Surg 2014;67:437-48. [Crossref] [PubMed]
- Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: A prospective comparative study J Plast Reconstr Aesth Surg 2013;66:1494-503.
- Wang CF, Zhou Z, Yan YJ, et al. Clinical Analyses of Clustered Microcalcifications after Autologous Fat Injection for Breast Augmentation. Plast Reconstr Surg 2011;127:1669-73. [Crossref] [PubMed]
- Chatterjee S, Laliberte M, Blelloch S, et al. Adipose-Derived Stromal Vascular Fraction Differentially Expands Breast Progenitors in Tissue Adjacent to Tumors Compared to Healthy Breast Tissue. Plast Reconstr Surg 2015;136:414e-25e. [Crossref] [PubMed]
- Kamat P, Schweizer R, Kaenel P, et al. Human Adipose-Derived Mesenchymal Stromal Cells May Promote Breast Cancer Progression and Metastatic Spread. Plast Reconstr Surg 2015;136:76-84. [Crossref] [PubMed]
- Kaoutzanis C, Xin M, Ballard TN, et al. Outcomes of Autologous Fat Grafting Following Breast Reconstruction in Post-Mastectomy Patients. Plast Reconstr Surg 2014;134:86-7. [Crossref]
- Seth AK, Hirsch EM, Kim JY, et al. Long-Term Outcomes following Fat Grafting in Prosthetic Breast Reconstruction: A Comparative Analysis. Plast Reconstr Surg 2012;130:984-90. [Crossref] [PubMed]
- Rubin JP, Coon D, Zuley M, et al. Mammographic Changes after Fat Transfer to the Breast Compared with Changes after Breast Reduction: A Blinded Study. Plast Reconstr Surg 2012;129:1029-38. [Crossref] [PubMed]
- Parikh RP, Doren EL, Mooney B, et al. Differentiating Fat Necrosis from Recurrent Malignancy in Fat-Grafted Breasts: An Imaging Classification System to Guide Management. Plast Reconstr Surg 2012;130:761-72. [Crossref] [PubMed]
- Rigotti G, Marchi A, Galiè M, et al. Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast Reconstr Surg 2007;119:1409-22; discussion 1423-4. [Crossref] [PubMed]
- Mineda K, Kuno S, Kato H, et al. Chronic inflammation and progressive calcification as a result of fat necrosis: The worst outcome in fat grafting. Plast Reconstr Surg 2014;133:1064-72. [Crossref] [PubMed]
- Khouri RK, Smit JM, Cardoso E, et al. Percutaneous aponeurectomy and lipofilling: A regenerative alternative to breast reconstruction. Plast Reconstr Surg 2013;132:1280-90. [Crossref] [PubMed]
- Choi M, Small K, Levowitz C, et al. The Volumetric Analysis of Fat Graft Survival in Breast Reconstruction. Plast Reconstr Surg 2013;131:185-91. [Crossref] [PubMed]
- Khouri RK, Rigotti G, Khouri RK, et al. Tissue-engineered breast reconstruction with Brava-assisted fat grafting: A 7-year, 488- patient, multicenter experience. Plast Reconstr Surg 2015;135:643-58. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. The evolution of breast implants. Clin Plast Surg 2009;36:1-13. v. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017;6:148-53. [Crossref] [PubMed]
- McLaughlin JK, Lipworth L, Murphy DK, et al. The safety of silicone gel-filled breast implants:a review of the epidemiologic evidence. Ann Plast Surg 2007;59:569-80. [Crossref] [PubMed]
- Spear SL, Murphy DK, Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle Round Silicone Breast Implants: Core Study Results at 10 Years. Plast Reconstr Surg 2014;133:1354-61. [Crossref] [PubMed]
- Maxwell GP, Van Natta BW, Bengston BP, et al. Ten-Year Results From the Natrelle 410 Anatomical Form-Stable Silicone Breast Implant Core Study. Aesthet Surg J 2015;35:145-55. [Crossref] [PubMed]
- Hammond DC, Migliori MM, Caplin DA, et al. Mentor Contour Profile Gel Implants: Clinical Outcomes at 6 Years. Plast Reconstr Surg 2012;129:1381-91. [Crossref] [PubMed]
- Cunningham B. The Mentor core study on silicone MemoryGel breast implants. Plast Reconstr Surg 2007;120:19S-29S; discussion 30S-2S.
- Cunningham B, McCue J. Safety and Effectiveness of Mentor’s MemoryGel Implants at 6 Years. Aesthetic Plast Surg 2009;33:440-4. [Crossref] [PubMed]
- Stevens WG, Harrington J, Alizadeh K, et al. Eight-Year Follow-Up Data from the U.S. Clinical Trial for Sientra’s FDA-Approved Round and Shaped Implants with High-Strength Cohesive Silicone Gel. Aesthet Surg J 2015;35:S3-10. [Crossref] [PubMed]
- Spear SL, Economides JM, Shuck J, et al. Analyzing implant movement with tabbed and nontabbed expanders through the process of two-stage breast reconstruction. Plast Reconstr Surg 2014;133:256e-60e. [Crossref] [PubMed]
- Ascherman JA, Zeidler K, Morrison KA, et al. Carbon Dioxide-Based versus Saline Tissue Expansion for Breast Reconstruction: Results of the XPAND Prospective, Randomized Clinical Trial. Plast Reconstr Surg 2016;138:1161-70. [Crossref] [PubMed]
- Montemurro P, Papas A, Heden P. Is Rotation a Concern with Anatomical Breast Implants? A Statistical Analysis of Factors Predisposing to Rotation. Plast Reconstr Surg 2017;139:1367-78. [Crossref] [PubMed]
- Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg 2013;132:1115-23. [Crossref] [PubMed]
- Nahabedian MY. Shaped versus round implants for breast reconstruction: indications and outcomes. Plast Reconstr Surg Glob Open 2014;2:e116. [Crossref] [PubMed]
- Clemens MW, Horwitz SM. NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J 2017;37:285-9. [Crossref] [PubMed]
- Loch-Wilkinson A, Beath KJ, Knight RJW. Breast implant associated anaplastic large cell lymphoma in Australia and New Zealand - high surface area textured implants are associated with increased risk. Plast Reconstr Surg 2017;140:645-54. [Crossref] [PubMed]
- Hu H, Johani K, Almatroudi A, et al. Bacterial Biofilm Infection Detected in Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast Reconstr Surg 2016;137:1659-69. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Bioengineered Breast: Concept, Technique, and Preliminary Results. Plast Reconstr Surg 2016;137:415-21. [Crossref] [PubMed]
- Salibian AA, Frey JD, Choi M, et al. Subcutaneous implant-based breast reconstruction with acellular dermal matrix/mesh: a systematic review. Plast Reconstr Surg Glob Open 2016;4:e1139. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Woo A, Harless C, Jacobson SR. Revisiting an old place: single surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J 2017;23:545-53. [Crossref] [PubMed]
- Schlenker JD, Bueno RA, Ricketson G, et al. Loss of silicone implants after subcutaneous mastectomy and reconstruction. Plast Reconstr Surg 1978;62:853-61. [Crossref] [PubMed]
- Sbitany H, Piper M, Pentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove N, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. PRSGO 2017;5:e1631. [PubMed]
- Rebowe RE, Allred LJ, Nahabedian MY. The Evolution from Subcutaneous to Prepectoral Prosthetic Breast Reconstruction. Plast Reconstr Surg Glob Open 2018;6:e1797. [Crossref] [PubMed]
- Ter Louw RP, Nahabedian MY. Prepectoral breast reconstruction. Plast Reconstr Surg 2017;140:51S-9S. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Staged Suprapectoral Expander/Implant Reconstruction without Acellular Dermal Matrix following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;139:30-9. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Vidya R, Iqbal FM. A Guide to Prepectoral Breast Reconstruction: A New Dimension to Implant-based Breast Reconstruction. Clin Breast Cancer 2017;17:266-71. [Crossref] [PubMed]
- Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77-86. [Crossref] [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast Reconstruction: Surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open 2016;3:e574. [Crossref] [PubMed]