Cryosurgery in combination with brachytherapy of iodine-125 seeds for pancreatic cancer
Introduction
Cryosurgery has been effectively used for treatment of a variety of tumors, such as prostate cancer, liver cancer and renal cancer (1), and is considered a promising modality for pancreatic cancer (2,3).
In vitro Studies on cancer cell lines (4) and in vivo animal studies on implanted tumors (5,6) suggest that temperatures of at least –40 °C may be necessary for adequate destruction of cancerous tissue, which is often used as a critical temperature in clinical practice (5). A major limit of cryoablation is incomplete destruction of cells in the border zone of the cryogenic lesion in which the tissue temperature is warmer than –20 °C. It is for this reason that adjunctive approaches are needed.
Adjunctive therapy, which may take the form of irradiation, cancer chemotherapeutic drugs, apoptotic promoters, or immunological potentiators have been shown to increase the efficacy of cryosurgery for many tumors (7).
External irradiation is usually regarded as insensitive to pancreatic cancer and associated with more systemic side effects (8) iodine-125 seed is an isotope which provides γ radiation for a short distance, resulting in the death of the targeted cells. Brachytherapy using iodine-125 seed implantation has been successfully used for the treatment of prostate cancer and metastatic or recurrent cancer (9,10). For more than 20 years, radioactive seed implantation has been used for treatment of pancreatic cancer, but its application alone appears to have a limited survival benefit for advanced cases (11,12).
Considering the advantages and disadvantages of each technique, it is presumed that the use of iodine-125 seed implantation is likely to be complementary to cryosurgery for treatment of pancreatic cancer. In this section, we mainly introduce the result of our studies on the combination treatment (3,13,14).
Indication
Theoretically, as for other cancer, cryosurgery is confined to treat for unresectable pancreatic cancer. However, as a matter of fact, there are few patients with pancreatic carcinoma who can be resected by operation, therefore, majority of pancreatic cancer should be treated with cryosurgery or cryosurgery in combination with pancreatic resection, if there is no contraindication (2,3,14). Obviously, these cases are adaptable for cryosurgery with combination of iodine-125 brachytherapy as well.
Technology
The procedure of cryosurgery and iodine-125 seed implantation is performed with percutaneous approaches.
Preoperative preparation
Patients fast for 24 h prior to the operation, and oral laxatives are given 12 h before the procedure. Patient is given medicine which inhibits pancreatic secretion 24 h before the operation to reduce the rates of complications. Sufficient breath training is given to ensure steady breath movement during the procedure. For patients with severe jaundice induced by obstruction of bile common duct, percutaneous transhepatic or endoscopic cholangiodrainage (PTCD) should be performed in order to improve symptoms and liver function.
Percutaneous cryosurgery
The procedure of percutaneous cryosurgery has already been introduced at Section “Cryosurgery for Pancreatic Cancer”. In brief, under local anesthesia and under guidance of ultrasound or CT, insertion of cryoprobe is performed through the peritoneal or retroperitoneal approach based on the location of the tumor. Generally, the 2- or 3-mm cryoprobe is used. For the tumor more than 3 cm in size, 2 to 3 probes may be used. For liver metastases, simultaneous cryosurgery is performed using additional cryoprobes which are inserted through right intercostal space. Once finishing the insertion of cryoprobes, two cycles of freezing/thawing are performed (3,13).
Iodine-125 seed implantation
Instruments
The iodine-125 seeds are manufactured from silver rods, which absorb iodine-125, and are enclosed in a titanium capsule welded by laser. Each seed source is 4-5 mm in length and about 1 mm in diameter (Figure 1). The iodine-125 produces gamma rays (5% of 35 keV, 95% of 28 keV) with a half-life of 59.6 days, half-value thickness of 0.025 mm of lead, penetration of 17 mm, incipient rate of 7 cGy/h, and activities of 0.5-0.8 mCi. Dose distribution was calculated using a brachytherapy planning system based on the American Association of Physicists in Medicine TG43 brachytherapy formalism (15). For the seed implantation 18-G implantation needles and turntable implantation gun are used.
The expected seed number implanted depends on the tumor volume. The total volume of each tumor is calculated according to the CT image with the treatment planning system (TPS) before implantation (16). The information from CT or MRI images is reconstructed into a three-dimensional form, and the precise margin of the tumor is outlined to facilitate the calculation of tumor matched peripheral dose (MPD). The number of implanted seeds is calculated according to the modified level formula (17). In practice, to reach the maximum radiation effect, the number of seeds implanted is 15% more than needed.
Seed implantation procedure
Seed implantation often is performed following cryoablation. Under CT or/and ultrasound guidance, the iodine-125 seeds are implanted into pancreatic tumor, mainly at the site of tumor near blood vessels and around the tumor border.
The distance between the adjacent implantation needles is approximately 1 cm each. It is to avoid puncturing vessels, pancreatic duct, and other nearby organs. Irruption of the stomach during the puncture generally does not result in substantial complications. However, intestine and colon should be avoided to be punctured especially when using large-bore needles. Repeated CT or ultrasound scanning with the implantation needles in place permitted adjustment of depth and angle of needle direction. Two to five seeds per needle are loaded, and seeds are released every 0.5-1 cm apart upon withdrawing the needles. Afterwards the implantation puncture site is bandaged and compressed to achieve hemostasis.
Clinical results
We (13,14) reported the results of cryosurgery and iodine-125 seed implantation for 49 patients with locally advanced pancreatic cancer in Fuda Cancer Hospital Guangzhou, China. Thirteen patients received intraoperative cryosurgery, and 36 patients underwent percutaneous cryosurgery. Among the patients that received percutaneous cryosurgery, 17 cases received the second session of cryosurgery, 3 cases received the third session of cryosurgery. Iodine-125 seeds implantation was performed during cryosurgery in 35 cases, and was done within the 3-9 days after cryosurgery in 14 cases. The number of iodine-125 seeds implanted for every patient was 34 in median with range of 18-54 seeds.
Response to treatment
According to the results of CT at 3 months after treatment, in most of the patients the tumors had varying degrees of necrosis, complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) were 20.4%, 38.8%, 30.6%, and 10.2%, respectively.
Overall survival
During a median follow-up of 18 months (range of 5-40 months), the median of overall survival was 16.2 months. Twenty-six patients (53.1%) had survival of 12 months or more, 8 had survival of 24 months or more. The patient with the longest survival is surviving without evidence of recurrence for 40 months (at the time of the article written, the patient has survival of 54 months). There were 36 patients of death, in whom 17 died of cancer spread, 11 hepatic metastases with liver failure, 5 cardio-cerebral vascular diseases, 3 unknown causes. The 6-, 12-, 24- and 36-month overall survival rates were 94.9%, 63.1%, 22.8%, and 9.5%.
There were 8 examples of the cases as follows:
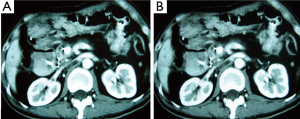
Case 1. Male, 80 years old. Ultrasound found an occupying lesion of 3 cm × 3 cm in size within pancreatic neck. Biopsy showed a cystadenocarcinoma. Patient underwent percutaneous cryosurgery with iodine-125 seed implantation under CT guidance. Three months after treatment, CT scan found that there was tumor necrosis, in which contained iodine-125 particles. Current ultrasound and CT scan shows that the original tumor was decreased to 1.5 cm × 1.1 cm in size (Figure 2). The patient has recurrence-free survival for 40 months.
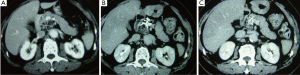
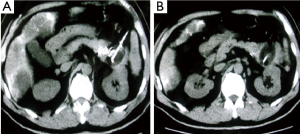
Case 2. Male, 61 years old. CT scan showed a low-density area of 4 cm × 5.5 cm in size in body of pancreatic and 3 intrahepatic lesions with sizes ranging from 2-5 cm. Biopsy showed adenocarcinoma. The Serum CA19-9 was 512 IU. The patient underwent percutaneous cryosurgery and iodine-125 seed implantation under guidance of CT/ultrasound for pancreatic lesion and hepatic metastases. Repeat CT scan showed the tumor shrinkage and stability of lesions in both pancreas and liver (Figure 3). Ultrasound-guided biopsy showed no evidence of cancer. CA19-9 levels were decreased to below 40 IU. The patient is alive for 27 months.
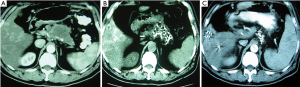
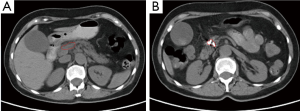
Case 3. Male, 36 years old. Ultrasound and CT revealed the mass of pancreatic head with dilated common bile duct. Serum CA19-9 was 210 IU/L. The patient underwent laparotomy which discovered the mass of 5 cm × 5 cm in size within pancreatic head. Biopsy showed moderate differentiation of adenocarcinoma. The palliative cholecystojejunostomy was performed for improvement of obstructive jaundice, and cryosurgery was performed for pancreatic tumor under direct vision and ultrasound guiding. CT follow-up at three months after the treatment showed the shrinkage and necrosis of the pancreatic mass with “honeycomb-like” change (Figure 4). CA19-9 was decreased to 48 IU. The patient had survival for 19 months.
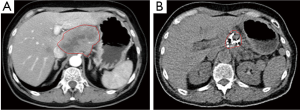
Case 4. Male, 67 years old, was detected to have obstructive jaundice and received CT examination, showing that the mass of 5 cm × 3 cm in size in pancreatic head and dilated common bile duct and gallbladder. He received percutaneous cryosurgery and iodine-125 seed implantation for pancreatic mass. Biopsy of mass showed moderately well-differentiated mucinous adenocarcinoma. CT at 8 months after treatment showed the shrinkage and necrosis of the pancreatic mass (Figure 5).
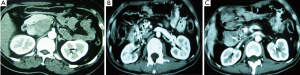
Case 5. Female, 59 years old. CT examination discovered a mass of 4 cm × 3 cm in size in pancreatic tail. Biopsy proved to be pancreatic adenocarcinoma. Percutaneous cryosurgery with iodine-125 seed implantation was performed (Figure 6). The follow-up at 14 months after treatment showed the stability of pancreatic tumor and the patient is surviving for 28 months.
Case 6. Female, 59 years old. Ultrasound and CT showed the mass of pancreatic head, 4 cm × 4 cm in size. She underwent percutaneous cryosurgery and iodine-125 seed implantation for pancreatic mass. Biopsy showed poor-differentiated adenocarcinoma. Twelve months later, the tumor of pancreatic head showed stability, however a new lesion developed in pancreatic body. She underwent second procedure of percutaneous cryosurgery for the lesion in pancreatic body. The follow-up using PET-CT at 3 months after the treatment showed a significant decrease of metabolic activity in original lesion (Figure 7). The patient had survival of 28 months.
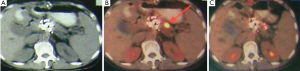
Case 7. Female, 61 years old. She has a biopsy-proved adenocarcinoma of pancreas. CT showed a 3 cm × 3 cm mass of pancreatic neck and 3 metastases in liver. Percutaneous cryosurgery and iodine-125 seed implantation were performed under CT guidance. At 3 months after treatment CT showed the shrinkage of pancreatic mass and disappearance of liver metastases (Figure 8). At 7 months after treatment PET-CT showed no evidence of pancreas and liver. She has survival of 27 months.
Case 8. Female, 64 years old. The ultrasound and CT examination discovered a big mass in pancreatic head. Biopsy of the mass showed squamous cell carcinoma. Percutaneous cryosurgery with combination of iodine-125 seed implantation for pancreatic mass was performed. At 8 months after treatment PET-CT showed mass had a significant shrinkage (Figure 9) and no metabolic activity. The patient has been alive for 14 months.
Adverse reactions
As showing in Table 1, 69.4% of patients had the abdominal pain, which subsided usually in 2-3 days. About half of the patients (51.0%) had an elevated serum amylase level, which generally ranged 1-2 times of the normal reference values for 5-7 days. Acute pancreatitis with acute abdominal pain, elevated serum amylase levels to four times or more was seen in 6 patients (12.2%), in whom one patient developed severe pancreatitis with intraabdominal fluid effusion, and serum amylase levels as high as 12 times of normal reference values. All patients with pancreatitis were cured by conservative management. Three patients (6.1%) had abdominal bleeding, however, the abdominal liquid drew out by paracentesis had no obvious increased levels of amylase activity. The bleeding was disappeared within four days. More than half of patients (53.1%) had fever of 38-39.5 °C, with temporary chill. Fever was persisted for 3-4 days, generally less than 7 days. Two patients had pulmonary infection, recovered with antibiotic therapy within 7-10 days. Two cases aged 78 and 91 years old, respectively, had cerebral infarction and myocardial infarction, respectively. No treatment-related mortality occurred.
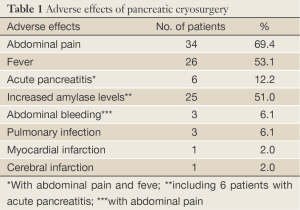
Full Table
Discussion
The rational of combination treatment
Iodine-125 seed implantation is a brachytherapy. Compared with other interventional procedures, this technique has several advantages, including: (I) radiation from seeds is characterized by attenuation over short distance outside the target area and low grade of depth dose, which can keep a higher accumulative dose within the tumor, increasing radiation dose to tumors without damaging neighboring organs (11); (II) with this technique, highly effective radiation doses are applied as a single fraction, ensuring protracted cell killing over a period of up to several weeks or months, causing tumor stem cell apoptosis, while fractionation of external radiotherapy is only effective in cells in some phases of cell cycle; (III) the traditional radiation has an inhomogeneous radiation absorption due to respiratory movements, while the radiation of radioactive seed is not affected by respiratory movements; (IV) low energy of radioactive seeds is able to decrease the metastasis of tumor by changing the immunophenotype of tumor cells.
During the past years, percutaneous image-guided radioactive seed implantation has been applied for treatment of diverse solid malignancies, which can be performed without surgery or general anesthesia has attracted increasing attention (18). However, there are few studies of this technique for pancreatic cancer with poor survival benefit (Table 2).
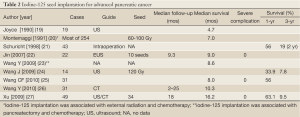
Full Table
Temperatures lower than –40 °C are assumed necessary to ensure the tumor ablation. The effectiveness of cryosurgery is dependent upon the completeness of cryoablation to all targeted tissue. The tumor persistence or occurrence at the site of cryoablation is often resulted from an incomplete destruction. Ice-balls larger than the target lesions are thus necessary for complete tumor ablation, because the outer few millimeters of the iceball circumference are at the non-lethal temperatures. The 1-cm ice-ball extension beyond the tumor borders should be often used as adequate for ablation (28,29). However, because the pancreas volume is relatively small, cancer often involves most of the glands, and over-freezing increases the complications, it is very difficult to ensure the “1 cm safe border”. Therefore, the combination of cryosurgery with iodine-125 seed brachytherapy for the treatment of the pancreatic cancer has been suggested. It is much more likely that iodine-125 seed implantation is complementary to cryosurgery.
Efficacy of combination treatment
Our study (3,13,14) showed that in patients with locally advanced pancreatic cancer, after the combination of cryosurgery with iodine-125 seed implantation. Most of the tumors had varying degrees of necrosis, CR, PR and SD were 20.4%, 38.8%, and 30.6%, respectively. The median survival was 16.2 months for all patients, more than 50% had survived 12 months or more. The overall 6-, 12-, 24- and 36-month survival rates were 94.9%, 63.1%, 22.8% and 9.5%, respectively.
In another study, we (30) have completed a prospective observation of percutaneous cryosurgery alone for locally advanced pancreatic cancer. The results showed that in all 59 patients, the median survival was 8.4 months. The overall survival at 3, 6 and 12 months was 89.7%, 61.1%, and 34.5%, respectively.
A comparison between both treatments shows that the 6- and 12-months survivals in patients who underwent combination are higher than that in patients who underwent cryosurgery alone, and median survival is longer in the former cases compared with the latter, as well. The results appear to be the result from the complimentary effect of iodine-125 implantation. It is important to note that in the patients with combination treatment there were 8 cases who survived for 24 mo or more. There is a patient with the longest survival who has recurrence-free survival of as long term as 54 months. The findings indicate that combination of cryosurgery and iodine-125 seed implantation offers the possibility of complete remission.
It is believed that tumor size is of critical importance in cryosurgery (30). However, tumor size could not be confirmed as an independent prognostic factor in our cases with combination treatment. This finding may be related to the possibility that the combination of cryosurgery and iodine-125 seed implantation may effectively destroy the entire tumor or a greater part of the targeted tissue, even in the presence of a large mass.
Safeness of combination treatment
In our series, there was no cryosurgery-related mortality. The main adverse effects were abdominal pain, fever and increased amylase levels. There were a few of the patients who developed acute pancreatitis, but all had good outcome. In addition, iodine-125 seed implantation, as a local therapy, may be performed at the same time of cryosurgery, and is not complicated by such persistent adverse effects as in chemo-radiotherapy (31,32). As a whole, the combination therapy consisted of cryosurgery and iodine-125 seed implantation is a less- or mini-invasive technique.
Conclusions
From our initial experience we conclude that the combination of percutaneous cryosurgery with combination of iodine-125 seed implantation appears to be a feasible, potentially safe and promising option in patients with locally advanced and unresectable pancreatic cancer, may result in longer survival and better quality of life of these patients. We now apply the combination treatment as the standard approach for management of the dismal disease. Nevertheless, a greater number of cases are needed to support our encouraging results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Gage AA, Baust JG. Cryosurgery - a review of recent advances and current issues. Cryo Letters 2002;23:69-78. [PubMed]
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009;37:783-805.
- Xu KC, Niu LZ, Hu YZ, et al. Combination of cryosurgery and 125iodine seed implantation for treatment of locally advanced pancreatic cancer. Technol Cancer Res Treat 2007;6:467-8.
- Baust JM, Vogel MJ, Van Buskirk R, et al. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplant 2001;10:561-71. [PubMed]
- Ohno K, Nelson LR, Mitooka K, et al. Transplantation of cryopreserved human corneas in a xenograft model. Cryobiology 2002;44:142-9. [PubMed]
- Hong JS, Rubinsky B. Patterns of ice formation in normal and malignant breast tissue. Cryobiology 1994;31:109-20. [PubMed]
- Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology 2009;59:229-43. [PubMed]
- Minsky BD, Hilaris B, Fuks Z. The role of radiation therapy in the control of pain from pancreatic carcinoma. J Pain Symptom Manage 1988;3:199-205. [PubMed]
- Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol 1995;153:1020-5. [PubMed]
- Holm HH, Juul N, Pedersen JF, et al. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. 1983. J Urol 2002;167:985-8; discussion 988-9. [PubMed]
- Peretz T, Nori D, Hilaris B, et al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys 1989;17:931-5. [PubMed]
- Takácsi-Nagy Z, Varga J, Poller I, et al. Successful treatment of a T1 cancer of the pancreatic head with high dose rate brachytherapy and external radiotherapy. Hepatogastroenterology 2002;49:844-6. [PubMed]
- Xu KC, Niu LZ, Hu YZ, et al. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J Dig Dis 2008;9:32-40. [PubMed]
- Xu KC, Niu LZ, Hu YZ, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol 2008;14:1603-11. [PubMed]
- Chen HH, Jia RF, Yu L, et al. Bystander effects induced by continuous low-dose-rate 125I seeds potentiate the killing action of irradiation on human lung cancer cells in vitro. Int J Radiat Oncol Biol Phys 2008;72:1560-6. [PubMed]
- Cengiz M, Gürdalli S, Selek U, et al. Effect of bladder distension on dose distribution of intracavitary brachytherapy for cervical cancer: three-dimensional computed tomography plan evaluation. Int J Radiat Oncol Biol Phys 2008;70:464-8. [PubMed]
- Monk BJ, Tewari KS, Puthawala AA, et al. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys 2002;52:806-15. [PubMed]
- Saito S, Nagata H, Kosugi M, et al. Brachytherapy with permanent seed implantation. Int J Clin Oncol 2007;12:395-407. [PubMed]
- Joyce F, Burcharth F, Holm HH, et al. Ultrasonically guided percutaneous implantation of iodine-125 seeds in pancreatic carcinoma. Int J Radiat Oncol Biol Phys 1990;19:1049-52. [PubMed]
- Montemaggi P, Dobelbower R, Crucitti F, et al. Interstitial brachytherapy for pancreatic cancer: report of seven cases treated with 125I and a review of the literature. Int J Radiat Oncol Biol Phys 1991;21:451-7. [PubMed]
- Schuricht AL, Spitz F, Barbot D, et al. Intraoperative radiotherapy in the combined-modality management of pancreatic cancer. Am Surg 1998;64:1043-9. [PubMed]
- Wang J, Jiang Y, Li J, et al. Intraoperative ultrasound-guided iodine-125 seed implantation for unresectable pancreatic carcinoma. J Exp Clin Cancer Res 2009;28:88. [PubMed]
- Wang Y, Zhao P, Wang CF, et al. Value of combined therapy in pancreatic cancer with a poor prognosis: analysis of 233 clinical cases. Zhonghua Yi Xue Za Zhi 2009;89:2381-5. [PubMed]
- Jin Z, Du Y, Li Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy 2008;40:314-20. [PubMed]
- Wang CF, Zhao P, Li YX, et al. Role of 125I seed implantation in treatment of unresectable pancreatic cancer. Zhonghua Yi Xue Za Zhi 2010;90:92-5. [PubMed]
- Zhongmin W, Yu L, Liu F, et al. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol 2010;20:1786-91. [PubMed]
- Xu KC, Niu LZ. Cryosurgery for Cancer. eds, In: Xu KC, Niu LZ, Hu YZ, et al. eds. Pancreatic cancer. Shanghai: Shanghai Science-Technology-Education Pub., 2007:234-45.
- Mala T, Samset E, Aurdal L, et al. Magnetic resonance imaging-estimated three-dimensional temperature distribution in liver cryolesions: a study of cryolesion characteristics assumed necessary for tumor ablation. Cryobiology 2001;43:268-75. [PubMed]
- Seifert JK, Gerharz CD, Mattes F, et al. A pig model of hepatic cryotherapy. In vivo temperature distribution during freezing and histopathological changes. Cryobiology 2003;47:214-26. [PubMed]
- Niu L, Li H, Xu K, et al. A novel cutaneous approach to deliver cryotherapy for the treatment of advanced pancreatic cancer, World Conference of Interventional Oncology-2010, Philadelphia, USA, 27.
- Maurer U, Wiegel T, Hinkelbein W, et al. Interstitial brachytherapy with permanent seed implants in early prostate cancer. Front Radiat Ther Oncol 2002;36:166-70. [PubMed]
- Al-Haj AN, Lobriguito AM, Lagarde CS. Radiation dose profile in 125I brachytherapy: an 8-year review. Radiat Prot Dosimetry 2004;111:115-9. [PubMed]