
Evaluating the current evidence to support therapeutic mammoplasty or breast-conserving surgery as an alternative to mastectomy in the treatment of multifocal and multicentric breast cancers
Introduction
The true incidence of multiple ipsilateral breast cancers (MIBCs) is currently unknown, however this is predicted to range from 20–25% following a recent systematic review of the English literature from May 1988 to July 2015 (1). The prevalence of MIBC varies from or 5–60% in published series (2-5). The term MIBC denotes either multifocal (MF) breast cancers that occur within close proximity separated by approximate radiological distances of 20–50 mm of normal tissue, or multicentric (MC) cancers occurring further apart in the breast at distances exceeding 50 mm (6). Historically these definitions have varied across studies resulting in clinical confusion, and confounding standardised study comparisons. The use of the term breast “quadrants” describing MF cancers within one “quadrant”, or MC cancers in separate “quadrants”, should now also be reconsidered, precluding inter-study reproducibility or diagnostic accuracy when informing the current management of MIBC (1). MF cancers comprise a far larger proportion of MIBC as reported in the EORTC AMAROS trial where they comprised 33% of cases (342/1,026) (7). Today, new radiological techniques such as magnetic resonance imaging (MRI) and enhanced mammography are increasing the diagnosis of MIBC, and underlining the importance of increasing our understanding of current evidence guiding treatment recommendations (8). Increasingly, emerging knowledge of breast cancer subtypes and associated heterogeneity in parallel with the launch of the UK Genomics 100k project and specialist research suggests that future definitions of MIBC may depend on the genomic homogeneity or heterogeneity between breast cancers, characterising either MF or MC MIBC, respectively (9). Therefore, future classifications of MIBC are likely to be genomic in nature, and comprise not only analyses of each cancer focus, but also of their surrounding stromal tissue (10). These exciting research areas will be further described under our biological understanding of MIBC. It is hoped that current and future high-quality studies and randomised trials in the UK, Europe and the USA will address some of these important issues (8).
MIBC breast cancer, what we know so far in relation to unifocal cancers
Although we anticipate increasing diagnoses of MIBC, little is known about their biological characteristics. Many retrospective studies have consistently shown a correlation between multifocality/multicentricity (MIBC) and the rate and extent of lymph node metastases (1,5). This is reported in 42% to 59% of cases (4,5,11,12), however this observation was not substantiated by the meta-analysis of Vera-Badillo et al. (13). Such varied observations may hypothetically relate to inconsistencies in TNM (tumor-node-metastasis) staging classifications, which fail to take account of global cancer volumes whose importance exceeds that of unifocal cancers. MIBC may more frequently be associated with poor prognostic factors compared with unifocal disease (14-18). These associations are potentially suggestive that MIBC are biologically more aggressive with a propensity for metastases. Whether MIBC is an independent adverse prognostic factor in breast cancer remains controversial. Considering the arbitrary distinction between multifocality and multicentricity, multiple simultaneous ipsilateral and synchronous lesions (MIBC) are now generally defined as multifocal breast cancers in the latest edition of the TNM classification, providing that they are macroscopically distinct and measurable using current traditional pathological and clinical tools (19). The latest version of the AJCC staging classification of tumour size (T) stipulates recording the largest diameter of the largest focus and not the summation of all foci, however, it recommends stipulating the code “m” is used to indicate multiple cancers or alternatively the total numbers of invasive cancers should be described, for example if the largest focus is 30 mm out of three invasive cancers then the T staging will be pathological (p) T2[3] (19). Furthermore, the biology of MIBC breast cancer is also now taken into consideration in the TNM classification, recommending evaluations of receptors [estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor (HER2)] on at least two foci or on all foci depending on morphological similarities or differences (19).
Coombs and Boyages (20) recommended using aggregate cancer dimensions, thereby upstaging most MIBC to more advanced stages, with rates of lymph node positivity stage-for-stage comparable to those of unifocal cancers. It is questionable whether the current TNM staging will amend its recommendations based on emerging high quality translational studies in future. Positive lymph node involvement was reported in 44–50% of MIBC cases, compared with 38% of unifocal cancers (7,14,15,17,20,21). Dual-localization sentinel lymph node biopsy (SLNB) is accurate diagnostically in MIBC (7). A subset of women with MIBC (342, 8.5%) in the European Organisation for Research and Treatment of Cancer 10981–22023 AMAROS (After Mapping of the Axilla: Radiotherapy or Surgery) trial had a 61% rate of SLNB positivity (105/171), compared with 28% of those with unifocal cancers (7,22). This suggests the safety of a sentinel lymph node biopsy in MIBC.
Some of the reviewed studies (16,23) reported worse disease-free survival (DFS) and overall survival for MIBC than for single cancers, yet other studies (24,25) noted similar outcomes. MC cancers (but not MF cancers) were distinguished by significantly worse overall (P=0.009) and DFS (P<0.001) compared with unifocal cancers (16). However, this was negated by a complete pathological response after neoadjuvant chemotherapy, and independent of type of surgery (16,26). Similarly, Wolters et al. (17) reported a significant association between MIBC and relapse-free survival in a study of 1,862 MIBC compared with 7,073 unifocal cancers (P=0.007); however, this finding related to clinical non-adherence to German guidelines. Weissenbacher and colleagues (27) confirmed a significant association between MIBC and overall breast cancer recurrence (P=0.001) in matched-pair multivariable analyses of MIBC compared with unifocal cancers (288 in each group). These conflicting reports support a future review of current TNM staging for MIBC. A meta-analysis by Vera-Badillo et al. (13) showed an apparent decreased overall survival in MIBC compared to unifocal cancers (HR =1.65, 95% CI: 1.07–2.52, P=0.02), without differences in recurrence free survival, however, the data are controversial in large part due to inter-study heterogeneity. MIBC (n=110) were independently predictive of local recurrence at 5 and 10 years compared to matched unifocal controls (n=263) independent of the type of surgery, albeit limited by a small retrospective study (HR =3.009, P=0.025) (28). Suggestions that unifocal cancers are biologically different from MIBC, renders the comparisons of breast conserving surgery (BCS) in MIBC to unifocal cancers as illogical and therefore is not an ideal evaluation on which to inform evidence-based treatment recommendations.
Biological features of MIBC cancers
MIBCs may arise secondarily to: intra-mammary spread of a single carcinoma, simultaneous outgrowth of independent cancer foci, or multiple carcinomas arising simultaneously via extensive intraductal or pre-invasive ductal carcinoma in situ (DCIS) (3). The third proposition more likely underlies the biological aetiology of MF cancers, few studies consolidate these theories by evaluating either histological or immunohistochemical characteristics of individual foci within MIBC (19). The majority of MIBC appear to be clonally related, potentially indicative of intramammary metastases reflecting a more aggressive phenotype compared to unifocal cancers (3). Initial observations demonstrated reasonable concordance of ER, PR and HER2 between multiple cancers in the breast (3). This implies that characterization of only one lesion, in the context of similar histological grades or receptor subtypes, should adequately individualise treatments (19). However, potential inter-lesional heterogeneity suggests that the exclusive characterization of the largest cancer focus is insufficient (29). Additional evidence shows that MIBC are associated with a significantly higher Nottingham Prognostic Index (NPI) compared to unifocal cancers (3,30). Proportionally, grade 1 histological cancers are also lower in MC cancers compared to MF and unifocal cancers, respectively (5). MF cancers were invasive ductal cancers with extensive DICS, compared to MC cancers that were invasive lobular as reported by Kanumuri et al. (5).
Molecular subtyping in breast cancers provides therapeutic and prognostic stratification (31,32). There is limited evidence on associations between MIBC and five molecular subtypes, compared with the subtype distribution in unifocal cancers (14). A comprehensive IHC subtyping algorithm (six biomarkers) that can distinguish luminal B from luminal A cancers, and basal from triple-negative disease, has potential clinical implications (32-34). Luminal cancers had a lower risk of 5-year LRR than HER2-positive or triple-negative unifocal disease after BCS in 12,500 patients (35). Ataseven and co-workers (16) reported increased associations between ER-positive and HER2-positive genotypes in MIBC, compared with unifocal cancers (P<0.001). Similarly, Moon et al. (36) reported fewer triple-negative MIBC than unifocal cancers. Lynch and colleagues (24) showed no significant associations between MIBC (906 patients) and molecular subtypes. Given the growing appreciation of intertumoral heterogeneity in MIBC, molecular characterization of a single focus may underestimate the molecular landscape (19). Standard phenotyping and genotyping of each cancer in MIBC should underpin future treatment recommendations (8,19).
Imaging for MIBC breast cancers
Clinically occult cancers may be treated adequately by adjuvant whole-breast RT after BCS (29). The incidence of clinically and radiologically detected MIBC ranges from 10% to 24% of all breast cancers (15-17,24), increasing with time from earlier to later studies. Two recent meta-analyses showed how MRI helped identify additional conventionally occult lesions in 15–27% of cases (37,38). This apparent doubling in incidence of MIBC over the 10 years between 1990 and 2000 may be due in part to improved breast imaging (digital mammography, ultrasonography and MRI) and increased screening (38). Standard imaging of MIBC should comprise digital mammography, ultrasound examination and MRI, with biopsy confirmation of any additional suspected cancers on MRI to minimize their misdiagnosis, which occurs in 30% of “lesions” (38,39), as recommended by the EUSOMA working guidelines.
Current evidence
The latest St Gallen consensus endorses the technical and cosmetic feasibility of treating some MIBC (MF and MC) with BCS, however, this endorsement is unsupported by high quality comparative clinical evidence to date, with no randomised trials or prospective cohort studies showing unequivocal oncological safety of BCS compared to mastectomy (40). Only six retrospective studies in a systematic review out of 24 eligible studies compared local regional recurrence (LRR) outcomes after BCS versus mastectomy (1,8). Remaining studies comprised seven case studies and thirteen studies evaluating the clinical outcomes of BCS for MIBC versus unifocal cancers (1,8). Two independent authors used the Newcastle Ottowa scoring system to assess study quality and showed these within the systematic review to be of poor to moderate quality (1). A meta-analysis was used to evaluate the rates of LRR after BCS compared to mastectomy in seven studies (1). A Forest plot of seven studies showed study homogeneity and apparently equivalent rates of LRR (risk ratio 0.94, 95% CI: 0.65–1.36) (1). This interpretation is contingent on extensive study limitations with the preferential selection of BCS for low risk cancers compared to mastectomy for aggressive cancers, consequently limiting the value of such a meta-analysis.
Observational studies evaluating treatments for MIBC have shown wide variation in clinical outcomes (1). There have also been wide ranging expert opinions on optimal surgical treatments (31,41). Inherent clinical inconsistencies include variable definitions, large variation in incidences depending on the sensitivity of preoperative imaging (for example mammography versus MRI), underestimating the tumour load using the current TNM staging classification and unknown clinical implications of MIBC, where MF cancers may be clinically and genetically distinguishable from MC ones (5,14,29).
Clinical cancer outcomes after breast-conserving surgery versus mastectomy for MIBC
Six (20,32-35,42) of seven studies reported clinical outcomes for BCS versus mastectomy for MIBC, which was the primary aim of the review, with a median follow-up of 59.5 (IQR, 56–81) months. The largest of the seven studies was part of the multicentre BRENDA cohort study (17), but did not provide raw data for comparison. This was scored as having moderate quality based on analyses of clinical subgroups, judged to be adherent to German guidelines or not. Adherence to guidelines meant that BCS was contraindicated for MC cancers (17). Non-conformance with guidelines resulted in 12.9% of MC cancers (60 of 464) being treated with BCS, compared with 46.8% (217 of 464) undergoing mastectomy (17). LRR was reported in five studies (15,42-45), distant metastases in three (15,42,43), overall survival in four (42-45) and DFS in two (17,44).
Local recurrence
Six studies (15,25,42-45) reported LRR rates ranging from 2% to 23% after BCS, with apparently similar rates of LRR for BCS compared with mastectomy. There was no heterogeneity in these studies, in part reflecting similar case selection biases with surgeons choosing BCS for low-risk patients and mastectomy for high-risk cases. Overall, the results are inconclusive and compromised by study quality.
The historical study of Yerushalmi and colleagues (25) reported the potential clinical equivalence of mastectomy in 887 patients compared with standard BCS in 300 patients, with 10-year LRR rates of 5.5% vs. 6.5% among 887 women undergoing mastectomy respectively (P=0.95). A significant limitation of this article was the lack of raw data comparing types of surgery in treating MIBC. Five-year LRR rates of MIBC in this study were 4.5% after mastectomy vs. 2.5% after BCS (25). This further attests to the limitations of clinician bias in the comparability of BCS to mastectomy in such studies.
Survival
Wolters and colleagues (17) concluded that treatment of MF cancers according to German guidelines by BCS (683 of 1,398, 48.9%) vs. mastectomy (329 of 1,398, 23.5%), showed no significant differences in 5-year recurrence-free survival. Neri and co-workers (15) showed that MF cancers were a significant independent predictor of worse breast cancer-specific survival for BCS (HR =3.88, 95% CI: 1.06–14.12, P=0.026) and mastectomy (HR =2.72, 95% CI: 1.15–6.48; P=0.023). Kadioğlu et al. (45) reported significantly better 5-year survival of 92% (median 95 months; range, 91–99 months) after BCS in 119 patients, compared with 72% (median 73 months; range, 68–78 months) after mastectomy in 103 patients (P<0.001). Multivariable analyses in the latter study, accounting for intergroup differences, subsequently showed no significant effects on outcomes between types of surgery (P=0.07) (45). Similarly, Kaplan and co-workers (42), Nos and colleagues (43) and Lim et al. (44) reported no differences in overall survival, DFS or distant metastases by type of surgery.
Current recommendations and practice
Two surveys of UK surgeons were conducted in collaboration with the Association of Breast Surgery in 2013 and 2015, respectively (personal communication ZE Winters). Ninety per cent of surgeons strongly expressed their support for a clinical trial addressing the clinical safety of BCS compared to mastectomy ± reconstruction in the treatment of MIBC. There appeared to be greater uncertainty regarding the optimal treatment of MF cancers, compared to assured recommendations for mastectomy in the case of MC cancers expressed by 80% of surgeons. Sixty to 70 per cent of surgeons reported their uncertainty about recommending BCS for MF cancers and were genuinely unconvinced by current evidence suggesting the comparable clinical safety of BCS to mastectomy. Based on the systematic review, surgeon’s surveys and Patient public opinion, a National Institute of Health Research funded (Research for Patient Benefit: PB-PG-1215-20009) randomised controlled trial called MIAMI will commence patient recruitment across multiple UK centres in 2018 (1,8). This is a feasibility study to evaluate the presence of collective clinical equipoise amongst patients and health care providers. The importance of the scientific questions is further underlined by an on-going USA prospective cohort study called the ACOSOG [American College of Surgeons Oncology Group) Z11102] (8,41). This cohort study is however limited in its comparability to outcomes relating to the current standard of care by excluding a control mastectomy arm.
Overall, there was limited evidence of moderate quality evaluating the clinical equivalence of BCS versus mastectomy for treating MIBC (1,8). Factors limiting the quality of evidence were study designs, heterogeneous clinical outcomes, and few if any representative studies of use of BCS to treat MC tumours compared with MF cancers. Most studies did not address the primary aim of the systematic review, but compared BCS for MIBC versus unifocal cancers (1). The apparent lack of significant intergroup differences in the rates of LRR support the rationale for a randomized trial. It is also poorly conceived to suggest that there is evidence of comparable rates of LRR reported by studies comparing BCS for MIBC to unifocal cancers (1). Despite this, Houvenaeghel et al. (4) and Nijenhuis et al. (3) in two recent reviews exclusively consider studies evaluating clinical outcomes following BCS for MIBC compared to unifocal cancers.
Currently the pathogenetic mechanisms underlying MIBC versus unifocal cancers are unknown (10,29). Despite uncertainties based on the evidence, Houvenaeghel et al. have recently suggested that the rate of local recurrences is usually low after BCS of MIBC, and proceed to propose that BCS is a reasonable option for MIBC (MF/MC) in women aged 50–69 years with small cancers and absence of extensive DCIS (4). In this article, the term breast conserving treatment (BCT) suggests the implicit adoption of the adjuvant medical treatments and radiotherapy producing synergistic benefits in clinical outcomes (4). In the systematic review (1), only two studies (16,26) described neoadjuvant chemotherapy. Modern neoadjuvant chemotherapy results in high rates of pathological complete responses in 60–70% of patients, especially in HER2 over-expressing and triple-negative cancers (46). Ataseven and colleagues (16) reported that neoadjuvant chemotherapy-induced pathological complete cancer response rates in MIBC increased the surgical options for BCS without compromising clinical outcomes, an approach requiring future investigation. In the absence of a pCR, MC cancers, but not MF cancers had a worse 3-year DFS (75.6% vs. 81.3%) and 3-year OS (84.7% vs. 88.2%) compared to unifocal cancers (P=0.009).
BCS with radiotherapy is widely accepted as an alternative to mastectomy in the treatment of early stage breast cancer (3,40). The 20-year follow-up of the National Surgical Adjuvant Breast and Bowel Project B-06 trial showed a local recurrence of 14.3% after BCS and radiotherapy (RT) (47). The effectiveness of boost RT to decrease LR has been established in a selected high-risk group of women aged 50 years or younger (48). In the systematic review, there was no mention of more than one lumpectomy bed receiving a tumour bed RT boost in MC cancers; however, there were only 223 MC cancers treated by BCS out of 3,537 women with MIBC (1). Consequently, there is currently no substantive evidence for the comparable safety and cosmetic acceptability of using a double RT boost after double lumpectomies in MC cancers compared to the EORTC boost trial (48). This question will be a novel area of investigation within the MIAMI trial (1,8). The feasibility of using double radiotherapy boosts will be evaluated for its safety and cosmetic results within the MIAMI trial (1,8). Bartelink et al. showed that boost RT in addition to 50 Gy of whole breast irradiation in unifocal cancers increases the 10-year rate of severe fibrosis from 1.6% to 4.4%, and of moderate fibrosis from 13% to 26% (48). Houvenaegehl et al. (4) conducted a dosimetric study to evaluate the volume of breast receiving an increased dose of RT in patients treated in the classical manner and in patients treated with a double boost. A second RT boost resulted in a 14% increase of the volume of breast receiving more than 55 Gy (from 19% to 33%), and a 10% increase of the volume of breast receiving more than 60 Gy (from 15% to 25%) (4). Overall, the ipsilateral whole breast received a mean 2 Gy increased dose of RT, whose clinical significance is unknown (4). Bracketing wires and 1,125 seeds should be used to localize and delineate the disease extent, applicable to either MF or MC cancers (49). There is a potential role for neoadjuvant chemotherapy or endocrine therapy if the involved breast cancer volume is too large, however there is a poor evidence base for BCS in this context, with no a priori randomised trials, other than the subset analysis within the GEPAR trials (16,46). The poorer prognostic implications of MIBC compared to unifocal cancers underlines the importance of adjuvant systemic treatments in all women, independent of the type of surgery. The MIAMI trial (1,8) proposes the prospective collection of patient reported quality of life questionnaires using the EORTC QLQ-C30, QLQ-BR23 and the EQ5D-5L (50) and standardised evaluations of cosmetic outcomes using the Breast Cancer Conservative Treatment cosmetic (BCCT.core) (51), which is a digital software program for evaluation of cosmetic outcomes previously validated in the TARGIT-A trial (52). Two validated quality of life questionnaires have been developed for women undergoing breast reconstruction called the BREAST-Q (50) and the EORTC BRECON23 (53), however neither have been robustly validated for therapeutic mammoplasty (TM) procedures using EORTC guidelines.
TM
The topographical localisation of MF or MC cancers must be considered in different regions of the breast and to the distance of the nipple-areolar complex (54). The new MIAMI trial has proposed a pragmatic distinction of either MF or MC cancers (Figure 1). MF cancers within the MIAMI trial will be defined as resettable by a single larger lumpectomy, compared to MC cancers definitively requiring at least two distinct lumpectomies (1). TM techniques comprise either extended breast tissue excisions for cancer(s) with simple re-approximation of breast tissue (level 1 re-coning) or a therapeutic reduction mammoplasty (level 2) (55,56). A comprehensive classification describing a breast quadrant per quadrant atlas for many oncoplastic surgical procedures has been proposed by Clough et al. (2010 and 2012, respectively) (57,58) reporting low re-operation rates, low risks of delayed adjuvant treatments and good cosmetic results. Clough et al. (59) also describe comparable rates of microscopically positive cancer margins following oncoplastic BCS (10/58, 17.2%) for MF versus unifocal cancers (23/217, 10.6%). Currently, TM is the standard best practice for optimizing cosmetic outcomes after extended breast tissue excisions relative to breast volume. Recently, a small case series (60) (68 patients) describing BCS for 20 patients with MF cancers was reported. In principle, treating MC cancers using two or more separate wide local excisions combined with TM merits future investigation, particularly in the context of RT boost(s) to one or more tumour beds. A meta-analysis (55) comparing 3,165 TM procedures with standard BCS in 5,494 patients with unifocal cancers showed that the former significantly reduced rates of cancer margin positivity (P<0.001) and surgical re-excisions (P<0.001) (61,62). Recently, the St Gallen panel (40) recommended a minimal acceptable surgical margin of “no ink on invasive tumour or DCIS”. Other interventions significantly reducing intraoperative tumour margin positivity have been described: digital specimen radiology (P=0.012 for digital vs. conventional mammography) (63), tumour margin cavity shaves (64) and real-time cancer margin assessments (19,65).
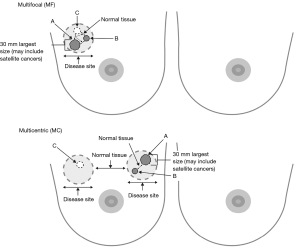
MF and MC breast cancer is regularly considered a relative contraindication for BCT (40). There are two reasons for this wide spread notion: (I) perceived higher risk for in-breast recurrence since it is assumed that in MF/MC cancer the risk of more invasive foci in the breast is greater, and therefore radiotherapy possibly less effective; (II) less good cosmetic outcome due to wider excisions, either segmental resection or quadrantectomy in multifocal, or multiple wide local excisions in MC disease. Mastectomy is therefore the treatment of choice for MF/MC breast cancer. Initial attempts at BCS for MF/MC breast cancer was met with little enthusiasm because early studies reported poorer local control for multiple ipsilateral breast lesions when compared with similar treatment for unifocal lesions (1). However, there is a growing body of evidence which suggests acceptable local control rates with MF/MC breast cancers treated with BCS, provided negative margins for each malignant focus and reasonable cosmetic outcomes (1,8). One of the possible barriers limiting BCT for MF/MC is the ability to fulfil guideline recommendations for the resection of all tumor foci through a single incision (57-59). MC lesions have either been treated with mastectomy or approached through two incisions (57-59). The latter approach may contravene current guidelines (66). Various oncoplastic techniques have been described to expand indications for BCS without significant compromise of cosmesis. Three factors are taken into consideration for surgical planning: skin and subcutaneous tissue, resection pattern and parenchymal repair (57-59). Thus, modifications of previously considered standard approaches might be necessary to allow a transition in treatment concept.
Current and future translational studies evaluating potential field defects
Identifying epigenetic changes in adjacent normal breast cancer tissues is likely to be important for understanding the aetiology of breast cancer, especially in the context of clinically diagnosed MIBCs (10). It remains unknown how epigenetic field defects comprising DNA methylation alterations contribute to carcinogenesis (10). Epigenetics is defined as heritable changes in gene regulation independent of DNA sequences. Direct effects on the DNA comprise the addition of a methyl group at cytosines of CG dinucleotides (commonly referred to as “CpGs”) (67). Teschendorff et al. analysed the DNA methylome of 569 breast tissue samples, including cancer free women (n=50) and matched normal cancer pairs (n=84) (10). Tens to thousands of epigenetic alterations comprised differentially variable methylated CpGs (DVMC cancers) that were more variable and hypermethylated in normal tissue (epithelial or stromal cell compartments) adjacent to breast cancers, including the cancers themselves (10). Normal tissues adjacent to breast cancers exhibited a significantly higher load of epigenetic changes field defects in stage-2 compared to stage-1cancers with adverse clinical outcomes (10). DVMC cancers identified cells that progressed to breast cancer with increased DVMC cancers in DCIS (10). The significant hotspots of epigenetic modulation mapped to the WNT (stem cell differentiation pathway) and FGF signalling pathways comprising promoter hypermethylation in the normal adjacent tissue surrounding breast cancers, unlike that in normal tissue from cancer-free women (10).
Future randomised clinical trials
A world-first randomised controlled trial called MIAMI (Safe Surgery for Multiple Breast Cancers) has been ethically approved in multiple UK centres (1,8). This will commence as a 3-year feasibility study and aims to recruit a total of 50 women with MIBC to evaluate the acceptability of women accepting the rationale for a trial based on current evidence, including their willingness to be randomised (1). The 1:1 randomisation will comprise allocation to either multiple lumpectomies and all types of TM (levels 1 and 2 including chest wall perforator flaps) or to the standard treatment of mastectomy and/or breast reconstruction (1,8). MIAMI is ethically approved and is a National Cancer Research Network (NCRN) portfolio study (1,8). The trial has proposed pragmatic definitions for MF or MC cancers and recommends diagnostic breast MRI alongside standard imaging (1,8) (Figure 1). A successful feasibility study will proceed to the main trial predicated on lessons learnt during the feasibility study. Importantly, the main MIAMI trial will evaluate the genomics of each breast cancer focus and their surrounding stromal tissues. In future, this diagnostic information may be fundamental in determining appropriate surgery recommendations based on cancer and/or stromal genomics. The MIAMI trial will evaluate individual patients requiring up to two lumpectomy radiotherapy boosts potentially recommended in some MC cancers (4). Dual boost RT is a novel therapeutic intervention using IMPORT HIGH and FAST FORWARD trial procedures with trial results pending, and demanding future assessments of cosmetic and patient reported outcomes (4,40,53).
Acknowledgements
Mr. Russell Davidson was responsible for design of Figure 1 which is original and not reproduced. Mr. Jonathan Horsnell, Ms. Rosemary Greenwood and Mr. Andrew Beswick assisted with the data analysis and interpretation.
Funding: This review underpins the MIHR funded MIAMI trial (PB-PG-1215-20009) and presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-0712-28073). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnote
Conflicts of Interest: Presented to the Association of Breast Surgery Conference, Bournemouth, UK, June 2015, and the San Antonio Breast Cancer Symposium, San Antonio, Texas, USA, December 2015; published in abstract form as Eur J Surg Oncol 2015;41:S37, and Breast 2016;26:149-150. ZE Winters is the Chief investigator of the NIHR funded MIAMI trial (Research for Patient Benefit: PB-PG-1215-20009) and also sits on the board of the European Organization of Research and Treatment of Breast Cancer (EORTC) and the international Society of Quality of Life (ISOQOL). L Bernaudo has no conflicts of interest to declare.
References
- Winters ZE, Horsnell J, Elvers KT, et al. Systematic review of the impact of breast-conserving surgery on cancer outcomes of multiple ipsilateral breast cancers. BJS Open 2018;2:162-74. [Crossref] [PubMed]
- Tan MP, Sitoh NY, Sitoh YY. Optimising Breast Conservation Treatment for Multifocal and Multicentric Breast Cancer: A Worthwhile Endeavour? World J Surg 2016;40:315-322. [Crossref] [PubMed]
- Nijenhuis MV, Rutgers EJ. Conservative surgery for multifocal/multicentric breast cancer. Breast 2015;24 Suppl 2:S96-9. [Crossref] [PubMed]
- Houvenaeghel G, Tallet A, Jalaguier-Coudray A, et al. Is breast conservative surgery a reasonable option in multifocal or multicentric tumors? World J Clin Oncol 2016;7:234-42. [Crossref] [PubMed]
- Kanumuri P, Hayse B, Killelea BK, et al. Characteristics of Multifocal and Multicentric Breast Cancers. Ann Surg Oncol 2015;22:2475-82. [Crossref] [PubMed]
- van la Parra RF, de Roos WK, Contant CM, et al. A prospective validation study of sentinel lymph node biopsy in multicentric breast cancer: SMMaC trial. Eur J Surg Oncol 2014;40:1250-5. [Crossref] [PubMed]
- Donker M, Straver ME, van Tienhoven G, et al. Comparison of the sentinel node procedure between patients with multifocal and unifocal breast cancer in the EORTC 10981-22023 AMAROS Trial: identification rate and nodal outcome. Eur J Cancer 2013;49:2093-100. [Crossref] [PubMed]
- Winters ZE, Benson MIAMI JR. (Multiple Ipsilateral breast conserving surgery versus mastectomy) Trial Management Group. Surgical treatment of multiple ipsilateral breast cancers. Br J Surg 2018;105:466-8. [Crossref] [PubMed]
- Genomics England, 100,000 Genomes project. Available online: https://www.genomicsengland.co.uk
- Teschendorff AE, Gao Y, Jones A, et al. DNA methylation outliers in normal breast tissue identify field defects that are enriched in cancer. Nat Commun 2016;7:10478. [Crossref] [PubMed]
- Goyal A, Newcombe RG, Mansel RE, et al. Sentinel lymph node biopsy in patients with multifocal breast cancer. Eur J Surg Oncol 2004;30:475-9. [Crossref] [PubMed]
- Spillane AJ, Brennan ME. Accuracy of sentinel lymph node biopsy in large and multifocal/multicentric breast carcinoma--a systematic review. Eur J Surg Oncol 2011;37:371-85. [Crossref] [PubMed]
- Vera-Badillo FE, Napoleone M, Ocana A, et al. Effect of multifocality and multicentricity on outcome in early stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2014;146:235-44. [Crossref] [PubMed]
- Bendifallah S, Werkoff G, Borie-Moutafoff C, et al. Multiple synchronous (multifocal and multicentric) breast cancer: clinical implications. Surg Oncol 2010;19:e115-23. [Crossref] [PubMed]
- Neri A, Marrelli D, Megha T, et al. Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases. BMC Surg 2015;15:1-9. [Crossref] [PubMed]
- Ataseven B, Lederer B, Blohmer JU, et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2015;22:1118-27. [Crossref] [PubMed]
- Wolters R, Wockel A, Janni W, et al. Comparing the outcome between multicentric and multifocal breast cancer: what is the impact on survival, and is there a role for guideline-adherent adjuvant therapy? A retrospective multicenter cohort study of 8935 patients. Breast Cancer Res Treat 2013;142:579-90. [Crossref] [PubMed]
- Yerushalmi R, Kennecke H, Woods R, et al. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat 2009;117:365-70. [Crossref] [PubMed]
- New Edition (7th) AJCC Staging System for breast cancer. Available online: http://labmed.ucsf.edu/uploads/210/101_new_ajcc_staging_of_breast_cancer_what_has_changed.pdf
- Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol 2005;23:7497-502. [Crossref] [PubMed]
- Schaverien MV, Stallard S, Dodwell D, Doughty JC. Use of boost radiotherapy in oncoplastic breast-conserving surgery - a systematic review. Eur J Surg Oncol 2013;39:1179-85. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Chung AP, Huynh K, Kidner T, et al. Comparison of outcomes of breast conserving therapy in multifocal and unifocal invasive breast cancer. J Am Coll Surg 2012;215:137-46. [Crossref] [PubMed]
- Lynch SP, Lei X, Hsu L, Meric-Bernstam F, et al. Breast cancer multifocality and multicentricity and locoregional recurrence. Oncologist 2013;18:1167-73. [Crossref] [PubMed]
- Yerushalmi R, Tyldesley S, Woods R, Kennecke HF, et al. Is breast-conserving therapy a safe option for patients with tumor multicentricity and multifocality? Ann Oncol 2012;23:876-81. [Crossref] [PubMed]
- Oh JL, Dryden MJ, Woodward WA, et al. Locoregional control of clinically diagnosed multifocal or multicentric breast cancer after neoadjuvant chemotherapy and locoregional therapy. J Clin Oncol 2006;24:4971-5. [Crossref] [PubMed]
- Weissenbacher TM, Zschage M, Janni W, et al. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat 2010;122:27-34. [Crossref] [PubMed]
- Shaikh T, Tam TY, Li T, et al. Multifocal and multicentric breast cancer is associated with increased local recurrence regardless of surgery type. Breast J 2015;21:121-6. [Crossref] [PubMed]
- Desmedt C, Fumagalli D, Pietri E, et al. Uncovering the genomic heterogeneity of multifocal breast cancer. J Pathol 2015;236:457-66. [Crossref] [PubMed]
- Joergensen LE, Gunnarsdottir KA, Lanng C, et al. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996-2001. Breast 2008;17:587-91. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736-50. [Crossref] [PubMed]
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [Crossref] [PubMed]
- Nielsen TO, Perou CM. CCR 20th anniversary commentary: the development of breast cancer molecular subtyping. Clin Cancer Res 2015;21:1779-81. [Crossref] [PubMed]
- Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012;133:831-41. [Crossref] [PubMed]
- Moon HG, Han W, Kim JY, et al. Effect of multiple invasive foci on breast cancer outcomes according to the molecular subtypes: a report from the Korean Breast Cancer Society. Ann Oncol 2013;24:2298-304. [Crossref] [PubMed]
- Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013;257:249-55. [Crossref] [PubMed]
- Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008;26:3248-58. [Crossref] [PubMed]
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010;46:1296-316. [Crossref] [PubMed]
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2018;29:2153. [Crossref] [PubMed]
- Boughey JC, Rosenkranz K, Nelson H. Multiple ipsilateral breast cancers: can the breast be preserved? Bull Am Coll Surg 2012;97:43-5. [PubMed]
- Kaplan J, Giron G, Tartter PI, et al. Breast conservation in patients with multiple ipsilateral synchronous cancers. J Am Coll Surg 2003;197:726-9. [Crossref] [PubMed]
- Nos C, Bourgeois D, Darles C, et al. Conservative treatment of multifocal breast cancer: a comparative study. Bull Cancer 1999;86:184-8. [PubMed]
- Lim W, Park EH, Choi SL, et al. Breast conserving surgery for multifocal breast cancer. Ann Surg 2009;249:87-90. [Crossref] [PubMed]
- Kadioğlu H, Yücel S, Yildiz S, et al. Feasibility of breast conserving surgery in multifocal breast cancers. Am J Surg 2014;208:457-64. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M. the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [Crossref] [PubMed]
- Harvey JR, Lim Y, Murphy J, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat 2018;169:531-6. [Crossref] [PubMed]
- Kanatas A, Velikova G, Roe B, et al. Patient-reported outcomes in breast oncology: a review of validated outcome instruments. Tumori 2012;98:678-88. [Crossref] [PubMed]
- Keshtgar MR, Williams NR, Bulsara M, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519-25. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Winters ZE, Afzal M, Rutherford C, et al. International validation of the European Organisation for Research and Treatment of Cancer QLQ-BRECON23 quality-of-life questionnaire for women undergoing breast reconstruction. Br J Surg 2018;105:209-22. [Crossref] [PubMed]
- Patani N, Carpenter R. Oncological and aesthetic considerations of conservational surgery for multifocal/multicentric breast cancer. Breast J 2010;16:222-32. [Crossref] [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- McIntosh J, O’Donoghue JM. Therapeutic mammoplasty - a systematic review of the evidence. Eur J Surg Oncol 2012;38:196-202. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Clough KB, Ihrai T, Oden S, et al. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg 2012;99:1389-95. [Crossref] [PubMed]
- Clough KB, Gouveia PF, Benyahi D, et al. Positive Margins After Oncoplastic Surgery for Breast Cancer. Ann Surg Oncol 2015;22:4247-53. [Crossref] [PubMed]
- Bamford R, Sutton R, McIntosh J. Therapeutic mammoplasty allows for clear surgical margins in large and multifocal tumours without delaying adjuvant therapy. Breast 2015;24:171-4. [Crossref] [PubMed]
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]
- Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer (Dove Med Press) 2017;9:521-30. [Crossref] [PubMed]
- Kim SH, Cornacchi SD, Heller B, et al. An evaluation of intraoperative digital specimen mammography versus conventional specimen radiography for the excision of nonpalpable breast lesions. Am J Surg 2013;205:703-10. [Crossref] [PubMed]
- Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. New Engl J Med 2015;373:503-10. [Crossref] [PubMed]
- Schnabel F, Boolbol SK, Gittleman M, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol 2014;21:1589-95. [Crossref] [PubMed]
- Association of Breast Surgery. Oncoplastic Breast Reconstruction Guidelines for Best Practice, 2014. Available online: http://www.associationofbreastsurgery.org.uk/media/23851/final_oncoplastic_guidelines_for_use.pdf
- Zheng SC, Widschwendter M, Teschendorff AE. Epigenetic drift, epigenetic clocks and cancer risk. Epigenomics 2016;8:705-19. [Crossref] [PubMed]


