Autologous reconstruction following nipple sparing mastectomy: a comprehensive review of the current literature
Background
Both oncologic surgical management of breast cancer and breast reconstruction have made significant advances over time. Breast cancer was described as early as the Edwin Smith papyrus (3000 BC), in which the ancient Egyptians concluded there was no cure for breast cancer (1). Roman physicians described surgical removal of breast tumors in the first century (2). In the modern surgical era, Halsted first performed his radical mastectomy—removal of all breast tissue, pectoralis major muscle, and associated lymphatic tissue—in 1882 (3). Now at the forefront of oncologic breast surgery is the nipple sparing mastectomy (NSM). It has been found to be oncologically safe in patients without skin involvement, nipple-areola complex (NAC) involvement, inflammatory breast cancer, or bloody nipple discharge (4).
Autologous breast reconstruction options have evolved over time as well. In 1887, Verneuil described reconstructing a breast with a superiorly based pedicle of breast tissue from the contralateral breast (5). In 1982, Hartrampf et al. first described the pedicled transverse rectus abdominis musculocutaneous (TRAM) flap for breast reconstruction (6). In 1979, Holmstrom first described abdominally-based free tissue transfer in breast reconstruction (7). And in 1989, the deep inferior epigastric artery perforator (DIEP) flap was described by Koshima and Soeda (8).
As surgical methods continue to evolve, and as patients become more educated consumers of their medical care, both breast surgeons and reconstructive plastic surgeons should understand the current options for both surgical resection and reconstruction in the treatment of breast cancer. This review will evaluate the current autologous breast reconstruction techniques following NSM.
Methods
The MEDLINE database was queried using both the OVID and PubMed user interfaces. Within OVID, the Medical Subject Headings (MeSH) “mastectomy”, “nipples”, and “surgical flaps” were exploded and combined with “and” modifiers, yielding 204 articles. Within PubMed, the search phrase “nipple sparing mastectomy autologous” yielded 149 results.
Potentially relevant abstracts were reviewed. Exclusion criteria were papers not in English, unavailable full texts, duplicates, or those that did not discuss autologous reconstruction after NSM. The remaining articles were thoroughly reviewed. Works cited within these papers, as well as supporting literature, were included within our review if deemed relevant.
Results
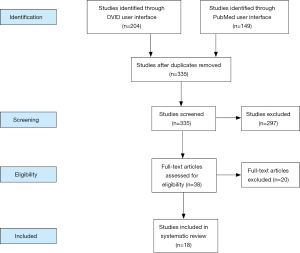
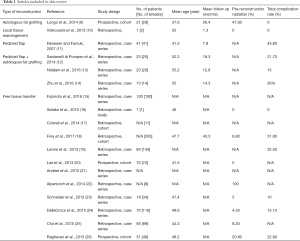
A total of 38 studies were included in the present review (Figure 1 and Table 1). Various rungs of the reconstructive ladder (27) have been used for autologous breast reconstruction following NSM. This results section will work its way up the ladder, with the majority of the review dedicated to free tissue transfer.
Full table
Autologous fat grafting
In 2014, Longo et al. published a 21-patient cohort study using fat grafting alone to reconstruct the breast following NSM (9). Their indications included: patient refusal of implant reconstruction, patients’ breasts were small or medium-sized, and there was a contraindication to free tissue transfer (e.g., insufficient donor site volume or previous surgeries precluding the use of common donor sites). Their surgical algorithm consisted of multiple fat grafting sessions, with a 3-month interval between each round of fat transfer. In non-irradiated patients, the first session was within 6 months of the oncologic resection. In irradiated patients, the group waited a minimum of 6 months after the completion of radiation before proceeding with fat grafting. Fat was harvested using a dry method, processed using centrifugation, and then was injected in small aliquots in the subcutaneous and submuscular planes. Intraoperative overcorrection was performed after the initial session, as the authors expected a 20–30% resorption rate. All non-irradiated patients had three rounds of fat grafting, while the irradiated patients required four to six sessions. As can be expected, the authors reported that the tight retracted skin in irradiated breasts limited the volume of fat able to be injected per session. No complications were reported from the fat grafting. Blinded, independent plastic surgeons rated results of the irradiated and non-irradiated breasts as 3.50 and 3.82, respectively, on the global aesthetic scale by Harris et al., in which 3 is good and 4 is excellent (28).
There has been concern that autologous fat grafting may increase patients’ risk of cancer recurrence. In vitro studies have shown that adipocytes can stimulate breast cancer cells via various mechanisms (29); however, no increased clinical risk has been demonstrated in recent literature. Myckatyn et al. published a multicenter, case-control study in the US in 2017 comparing 225 patients with recurrence following immediate breast reconstruction with 972 random controls (30). The subset of patients reconstructed with fat grafting had an equivalent risk of recurrence compared to other patients, even after controlling for other variables. Petit et al. performed a cohort study in 2017 with 322 patients who had fat grafting reconstruction following oncologic surgery for invasive breast cancer (31). These were compared to a group of individually matched patients who did not receive fat grafting. No difference was seen between these two groups for local recurrence or distant metastases.
Local tissue rearrangement
In 2012, Richardson and Ma first described the Goldilocks mastectomy (32). This surgery begins with a skin sparing mastectomy performed via circumareolar or elliptical skin incisions. The redundant mastectomy skin within the markings of a standard Wise skin-reduction pattern (33) is deepithelialized and then used to create the new breast mound. In 2013, Vrekoussis et al. published a case report of a patient treated with bilateral NSM and reconstructed in a fashion similar to the Goldilocks mastectomy, except with preservation of the NAC (10). The patient had large, severely ptotic breasts. The breast tissue resection was accessed through the superior aspect of the markings, with the (NAC maintained on an inferiorly-based dermal-subcutaneous pedicle. Due to a significant amount of fat remaining on the mastectomy skin flaps, the dermal-subcutaneous pedicles were able to reconstruct the breast mounds with a reasonable amount of volume. The only postoperative complication reported was transient bruising.
In 2016, Schwartz and Skowronski described a case of a patient with large ptotic breasts on whom they performed bilateral Goldilocks mastectomies with free NAC grafting (34). As these procedures rely solely on the volume of the mastectomy skin flaps, patients with small breasts and/or minimal ptosis are poor candidates for this type of reconstruction. In 2017, Schwartz and Skowronski suggested adding a second stage to their Goldilocks mastectomy with free NAC grafting in patients with moderate breast size (35). After the initial procedure, triangles of skin along the vertical limbs were deepithelialized and involuted, and fat grafting to the upper pole and retroareolar regions was performed to increase volume and improve shape.
Pedicled tissue
Pedicled flaps based on the thoracodorsal artery—either the latissimus dorsi (LD) muscle flap (with or without a skin paddle) or the thoracodorsal artery perforator (TDAP) flap—are commonly used to augment soft tissue coverage in implant-based breast reconstruction. With modifications to increase flap volume, these pedicled flaps have also been described in the setting of NSM (36), and may be used for total autologous breast reconstruction.
In 2007, Denewer and Farouk published their results from a case series of 41 patients reconstructed with unilateral, extended LD muscle flaps immediately following NSM (11). The incision for the NSM, described as “claw-like” along the lateral half of the areola, with a lateral radial extension towards the axilla, allowed access to the thoracodorsal vessels. The pedicle was dissected along its origin at the subscapular artery towards its bifurcation. Both the dorsal branch supplying the LD muscle and the thoracic branch supplying the serratus anterior were included, and some of the subcutaneous fat was maintained on the muscle bellies in order to maximize volume. To ensure full mobilization, the LD was disinserted from the humerus, transposed to the anterior chest and the subcutaneous fat layer laid over the pectoralis major muscle. The LD muscle was then folded on itself before redraping the mastectomy skin flaps. No additional incisions were required on the back. Of the 41 patients, 90.2% had reconstructions independently rated as good or excellent at the 6-week postoperative mark. The most common complication in 14.6% of patients was donor site seroma formation, a well-known occurrence after the harvest of LD flaps.
In light of the potential for inadequate long-term reconstructed breast volume after atrophy of the LD flap, concurrent fat grafting can be performed. In 2014, Santanelli di Pompeo et al. described their technique of immediate fat grafting into the LD fascia and overlying adipose layers (12). In 23 patients (25 breasts), they injected an average of 101 mL of fat and reported no complications. In 2016, Niddam et al. reported fat injections into the pectoralis major muscle at the time of a pedicled LD flap in 20 patients undergoing unilateral breast reconstruction (13). Their mean volume of fat injected was 228 mL. Two patients required a secondary fat grafting procedure to correct asymmetry due to inadequate breast reconstruction volume. Zhu et al. reported using a multisite fat grafting technique at the time of a pedicled LD reconstruction (14). In 14 breasts (10 patients), an average of 176 mL of fat was distributed into the LD muscle, the adipose tissue overlying the LD, the mastectomy skin flaps, the pectoralis major muscle, and the serratus anterior muscle. Three patients required additional fat grafting.
Free tissue transfer
Free tissue transfer is the most common method of autologous reconstruction following NSM. Donor sites typically include the lower abdomen, buttocks, or thighs (posterior and medial), and the internal mammary or thoracodorsal systems most commonly serve as adequate recipient vessels. Fujimoto et al. described their donor site selection algorithm after NSM and skin sparing mastectomies (15). Abdominally based flaps were avoided in patients desiring future pregnancies. In this cohort, posteromedial thigh flaps were used if the breast size was B cup or smaller. Superior and inferior gluteal artery perforator flaps were used if the tissue volume at the posterior thigh donor site was insufficient.
Satake et al., in 2016, reported a unique case of reconstruction following NSM in a patient with inadequate abdominal, thigh, or buttock donor sites. An adipofascial flap based on a lumbar artery perforator (which was visualized on preoperative imaging) was harvested and anastomosed to the lateral thoracic vessels. A vein graft was required during the arterial anastomosis due to lack of pedicle length and a size mismatch (16).
Discussion
Type of incision
The type of incision used for NSM followed by autologous reconstruction needs to satisfy multiple criteria. It should preserve perfusion to the NAC, provide sufficient access to the recipient vessels for microvascular anastamosis, and allow for good cosmesis.
The perfusion of the NAC has been shown to depend on the type of incision used. In 2014, Colwell et al. analyzed their outcomes after reconstruction of 500 NSM based on incision type (17). Periareolar incisions were associated with the highest rate of total complications (21.1%) and NAC necrosis (10.5%). Inferolateral inframammary fold incisions were associated with the lowest total complications (8.5%) and NAC necrosis (0.8%).
In a 2017 study, Frey et al. analyzed the outcomes of 1,028 NSM reconstructions—both autologous and implant-based (18). While the most common incision utilized for implant-based reconstruction was along the inframammary fold, the vertical incision was most commonly used during autologous reconstruction. This vertical, infra-areolar incision was preferred by reconstructive surgeons, as it allowed for easier access to the internal mammary vessels while maintaining good cosmesis, especially after subsequent excision of the skin paddle.
In 2013, Levine et al. described their experience with NSM incision-types when reconstructing breasts with completely buried free flaps (19). While the lateral incision was easiest for the oncologic surgeons, the reconstructive team preferred an inframammary incision for small breasts with minimal ptosis and a vertical incision for larger, more ptotic breasts. Secondary reductions and mastopexies allowed incorporation of this vertical scar.
Autologous versus prosthetic based reconstruction
Rates of autologous versus prosthetic reconstruction following NSM are highly variable, with institutional rates of autologous reconstruction as low as 2% (17), and as high as 54% (20). Findings have been mixed regarding the comparative complication rates of the two reconstructive modalities. In 2017 Frey et al. analyzed 1,028 NSM (18). A total of 51.8% (n=533) were reconstructed using tissue expanders, 25.6% (n=263) autologous-based, and 22.6% (n=232) direct-to-implant. Both the tissue expander and direct-to-implant groups had higher rates of minor cellulitis (P=0.0002 and 0.0051, respectively). The tissue expander group had less complete nipple necrosis and major mastectomy flap necrosis than the autologous-based group (P=0.0126 and <0.0001, respectively). The direct-to-implant group had higher rates of minor mastectomy flap necrosis and partial nipple necrosis compared to the autologous-based group (P=0.0425 and 0.0437, respectively).
In 2013 Lee et al. reviewed 130 NSM, 70 reconstructed using autologous tissue and 60 utilizing tissue expander-based reconstructions (20). The mastectomy skin flap necrosis rate was significantly lower in the autologous-based group in both univariate and multivariate analyses (P=0.034 and 0.024, respectively).
Prior irradiation
Special consideration should be given during autologous reconstruction following NSM in previously irradiated breasts. In a 2012 study by Andree et al., 64 immediate free flap reconstructions following both skin- and nipple-sparing mastectomies were performed in patients who had undergone prior breast conservation therapy (BCT) (21). Either DIEP or muscle sparing-2 TRAM flaps were used. Due to radiation-induced fibrotic skin changes, they externalized a skin island, even in the setting of an NSM, to expand the skin envelope. The total flap loss rate was higher in the previous BCT group (1.6% compared to 0.8%), as was the partial flap loss rate (3.1% compared to 1.3%).
In 2014, Alperovich et al. published their outcomes of both implant-based and autologous reconstruction following NSM in 26 previously irradiated breasts (24 patients) (22). Nine of these breasts were reconstructed using autologous flaps and the remainder using tissue expander/immediate implant reconstruction (16/26) and LD flap with an implant (1/26). The rate of mastectomy skin flap necrosis that required reoperation was 11.5% (3/26), with one of these a free tissue transfer reconstruction requiring debridement and split thickness skin grafting. The other two cases were implant based reconstructions that required explantation. They compared these complication rates to 330 non-irradiated NSM breast reconstructions and found no statistically significant differences; however, the rate of implant or tissue expander explantation in the cohort with previous irradiation (2/26, 7.7%) was trending towards significance (P=0.06), and the true rate of explantation was underestimated as the nine autologous reconstructions were included in the denominator.
NSM in the large, ptotic breast
In 2006, Sacchini et al. reported that they counseled patients with large, ptotic breasts against NSM—citing risks of nipple necrosis and asymmetry (37). Since then, multiple techniques have been cited in the literature for optimizing outcomes of NSM in this breast type: pre-mastectomy delay procedures, pre- or post-mastectomy mastopexies, free NAC graft, and post-mastectomy hyperbaric oxygen therapy (38-43).
A 2012 study by Schneider et al. reported their experience with autologous reconstruction in patients with large and ptotic breasts who had not undergone any pre-mastectomy delay (23). Nineteen patients (34 breasts) met inclusion criteria: cup size C or greater, sternal notch to nipple distance >24 cm, and Regnault grade II or III breast ptosis (44). There was 1 case (5%) of nipple necrosis in a breast that was previously irradiated, 1 (5%) hematoma, and no partial or complete flap loss. Five patients (26%) underwent secondary mastopexies at an average of 6.6 months after their initial surgery without any complications. The blood supply to the NAC was bolstered due to neovascularization from the underlying flap, compared to the relatively tenuous blood supply to the NAC at the time of the NSM. And, in contrast to pre-mastectomy mastopexies, the oncologic surgical management wasn’t delayed in these patients. The authors believe that their success with the large, ptotic breast was attributable to oncologic surgeon dependent factors (not dissecting beyond the borders of the breast and preserving subcutaneous fat and the blood supply from the second intercostal perforator) as well as favorable patient demographics (no smokers or diabetics).
A 2015 study by DellaCroce et al. corroborated the finding that secondary mastopexies can be performed safely after NSM with autologous reconstruction (24). Seventy patients with 116 NSM followed by perforator flap reconstruction had secondary mastopexy procedures for grade II or III ptosis. They used full-thickness periareolar incisions and undermining of the surrounding breast skin flaps. There were no cases of postoperative NAC necrosis. The most common complications were partial incisional dehiscence (n=9, 7.8%) and partial mastectomy flap necrosis (n=4, 3.4%).
NAC necrosis management
NAC necrosis is one of the potential complications in NSM. An advantage of autologous tissue reconstruction is the option of banking the flap skin paddle to be used to resurface necrotic NAC or mastectomy flaps (25). In 2015, Cho et al. described their method of preserving a circle of flap skin paddle, approximately 5 cm in diameter, underneath the NAC (45). When NAC necrosis was observed postoperatively, the NAC was debrided and the flap skin paddle externalized. In cases in which the NAC survived, the team went back to the operating room 2 weeks after the initial reconstruction and de-epithelialized the skin paddle. The authors recommend using this method if intraoperative signs of NAC ischemia are noted or if patients have at least two of four preoperative risk factors they found to be significant for NAC necrosis: BMI ≥25, smoking, preoperative radiation, and large breast size.
Single stage reconstruction
A 2013 study by Levine et al. described their experience with immediate breast reconstruction using buried free flaps following NSM with the goal of a single stage reconstruction (19). Risk factors for mastectomy flap necrosis—smoking, prior radiation, and connective tissue disorders—were relative contraindications. Additionally, buried flaps were only used when the oncologic surgeon was known to leave healthy mastectomy flaps. Eighty-four patients and 134 buried flaps were reported in the series—all monitored with Cook implantable Dopplers. A total of 3.0% of flaps (n=4) were re-explored for venous obstruction, and 2.2% of flaps (n=3) ultimately failed. Delayed mastectomy flap healing occurred in 3.7% of breasts (n=5) which were managed with local wound care. At the time of publication, 58.2% (n=78) of their breasts had secondary surgeries for cosmesis, while 41.8% (n=56) had no revisions.
While this study reports low complication rates, a 2015 study by Raghavan et al. supports a two-staged reconstruction (26). In their experience, immediate autologous free flap reconstruction after NSM was associated with ischemic complications of the mastectomy skin flap and NAC. Therefore, in 2013, the authors started using a two-stage technique with insertion of a tissue expander at the time of the NSM, followed by tissue expander removal and autologous free flap reconstruction at an average of 11.5 months later. Their NAC necrosis rate dropped from 29% to 0%. Additionally, NAC overall appearance and color in the delayed cohort were rated as significantly better by blinded surgeons and residents using a five-point Likert scale.
Conclusions
Autologous breast reconstruction after NSM is a safe and aesthetic method of breast cancer surgical management. Autologous reconstruction can be effectively performed using multiple rungs of the reconstructive ladder: autologous fat grafting, local tissue rearrangement, pedicled flaps, and free tissue transfer. Superiority of autologous versus prosthetic reconstruction has yet to be conclusively shown. Special consideration should be given to previously irradiated breasts, as well as large, ptotic breasts. Outcomes-based research is critical to continue to improve surgical care for this deserving patient population.
Acknowledgements
This work was supported by the Division of Plastic Surgery at the Mount Sinai Health System.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cooper WA. The history of radical mastectomy. Ann Med Hist 1941;3:36-54.
- Donegan WL. Introduction to the history of breast cancer. Cancer of the Breast. Philadelphia: WB Saunders, 1995.
- Halsted WS. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555. [Crossref] [PubMed]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Verneuil A. Memoires de chirurgie, Paris, 1887.
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [Crossref] [PubMed]
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. Scand J Plast Reconstr Surg 1979;13:423-7. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Longo B, Laporta R, Sorotos M, et al. Total breast reconstruction using autologous fat grafting following nipple-sparing mastectomy in irradiated and non-irradiated patients. Aesthetic Plast Surg 2014;38:1101-8. [Crossref] [PubMed]
- Vrekoussis T, Perabo M, Himsl I, et al. Bilateral prophylactic skin-reducing nipple-sparing mastectomy with immediate breast reconstruction using only a vascularized dermal-subcutaneous pedicle: technique and possible advantages. Arch Gynecol Obstet 2013;287:749-53. [Crossref] [PubMed]
- Denewer A, Farouk O. Can Nipple-sparing Mastectomy and Immediate Breast Reconstruction with Modified Extended Latissimus Dorsi Muscular Flap Improve the Cosmetic and Functional Outcome among Patients with Breast Carcinoma? World J Surg 2007;31:1169-77. [Crossref] [PubMed]
- Santanelli di Pompeo F, Laporta R, Sorotos M, et al. Latissimus Dorsi Flap for Total Autologous Immediate Breast Reconstruction without Implants. Plast Reconstr Surg 2014;134:871e-9e. [Crossref] [PubMed]
- Niddam J, Vidal L, Hersant B, et al. Primary Fat Grafting to the Pectoralis Muscle during Latissimus Dorsi Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e1059. [Crossref] [PubMed]
- Zhu L, Mohan AT, Vijayasekaran A, et al. Maximizing the Volume of Latissimus Dorsi Flap in Autologous Breast Reconstruction with Simultaneous Multisite Fat Grafting. Aesthet Surg J 2016;36:169-78. [Crossref] [PubMed]
- Fujimoto H, Ishikawa T, Satake T, et al. Donor site selection and clinical outcomes of nipple-areola skin-sparing mastectomy with immediate autologous free flap reconstruction: A single-institution experience. Eur J Surg Oncol 2016;42:369-75. [Crossref] [PubMed]
- Satake T, Nakasone R, Kobayashi S, et al. Immediate breast reconstruction using the free lumbar artery perforator flap and lateral thoracic vein interposition graft for recipient lateral thoracic artery anastomosis. Indian J Plast Surg 2016;49:91-4. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast Reconstruction following Nipple-Sparing Mastectomy: Predictors of Complications, Reconstruction Outcomes, and 5-Year Trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- Frey JD, Choi M, Salibian AA, et al. Comparison of Outcomes with Tissue Expander, Immediate Implant, and Autologous Breast Reconstruction in Greater Than 1000 Nipple-Sparing Mastectomies. Plast Reconstr Surg 2017;139:1300-10. [Crossref] [PubMed]
- Levine SM, Snider C, Gerald G, et al. Buried Flap Reconstruction after Nipple-Sparing Mastectomy: Advancing toward Single-Stage Breast Reconstruction. Plast Reconstr Surg 2013;132:489e-97e. [Crossref] [PubMed]
- Lee KT, Pyon J, Bang S, et al. Does the reconstruction method influence development of mastectomy flap complications in nipple-sparing mastectomy? J Plast Reconstr Aesthet Surg 2013;66:1543-50. [Crossref] [PubMed]
- Andree C, Munder B, Seidenstuecker K, et al. Skin-sparing mastectomy and immediate reconstruction with DIEP flap after breast-conserving therapy. Med Sci Monit 2012;18:CR716-20. [Crossref] [PubMed]
- Alperovich M, Choi M, Frey JD, et al. Nipple-Sparing Mastectomy in Patients with Prior Breast Irradiation: Are Patients at Higher Risk for Reconstructive Complications? Plast Reconstr Surg 2014;134:202e-6e. [Crossref] [PubMed]
- Schneider LF, Chen CM, Stolier AJ, et al. Nipple-Sparing Mastectomy and Immediate Free-Flap Reconstruction in the Large Ptotic Breast. Ann Plast Surg 2012;69:425-8. [Crossref] [PubMed]
- DellaCroce FJ, Blum CA, Sullivan SK. Nipple-Sparing Mastectomy and Ptosis: Perforator Flap Breast Reconstruction Allows Full Secondary Mastopexy with Complete Nipple Areolar Repositioning. Plast Reconstr Surg 2015;136:1e-9e. [Crossref] [PubMed]
- Kovach SJ, Georgiade GS. The "banked" TRAM: a method to insure mastectomy skin-flap survival. Ann Plast Surg 2006;57:366-9. [Crossref] [PubMed]
- Raghavan S, Warren Peled A, Hansen SL, et al. Approaches to Microvascular Breast Reconstruction After Total Skin-Sparing Mastectomy. Ann Plast Surg 2015;74 Suppl 1:S46-51. [Crossref] [PubMed]
- Simman R. Wound Closure and the Reconstructive Ladder in Plastic Surgery. J AM Col Certif Wound Spec 2009;1:6-11. [Crossref] [PubMed]
- Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257-61. [Crossref] [PubMed]
- Lohsiriwat V, Curigliano G, Rietjens M, et al. Autologous fat transplantation in patients with breast cancer: “silencing” or “fueling” cancer recurrence? Breast 2011;20:351-7. [Crossref] [PubMed]
- Myckatyn TM, Wagneer IJ, Mehrara BJ, et al. Cancer Recurrence After Fat Transfer (CRAFT)- A Multicenter Case-Cohort Study. Plast Reconstr Surg 2017;139:11-8. [Crossref] [PubMed]
- Petit JY, Maisonneuve P, Rotmensz N, et al. Fat Grafting after Invasive Breast Cancer: A Matched Case-Control Study. Plast Reconstr Surg 2017;139:1292-6. [Crossref] [PubMed]
- Richardson H, Ma G. The Goldilocks mastectomy. Int J Surg 2012;10:522-6. [Crossref] [PubMed]
- Robbins TH. A reduction mammaplasty with the areola-nipple based on an inferior dermal pedicle. Plast Reconstr Surg 1977;59:64-7. [Crossref] [PubMed]
- Schwartz JC, Skowronski PP. Total Single-Stage Autologous Breast Reconstruction with Free Nipple Grafts. Plast Reconstr Surg Glob Open 2016;3:e587. [Crossref] [PubMed]
- Schwartz JD, Skowronski PP. Extending the Indications for Autologous Breast Reconstruction Using a Two-Stage Modified Goldilocks Procedure: A Case Report. Breast J 2017;23:344-7. [Crossref] [PubMed]
- Al-Khyatt W, Goyal A, Mansel RE. Nipple-Sparing Skin-Sparing Mastectomy and Vertical Latissimus Dorsi Flap Reconstruction for Bilateral Fibromatosis of the Breast. Clin Breast Cancer 2010;10:E1-2. [Crossref] [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [Crossref] [PubMed]
- Spear SL, Hannan CM, Willey SC, et al. Nipple-Sparing Mastectomy. Plast Reconstr Surg 2009;123:1665-73. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-Sparing Mastectomy for Prophylactic and Therapeutic Indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Karian LS, Therattil PJ, Wey PD, et al. Delay techniques for nipple-sparing mastectomy: A systematic review. J Plast Reconstr Aesthet Surg 2017;70:236-42. [Crossref] [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical Delay of the Nipple-Areolar Complex: A Powerful Technique to Maximize Nipple Viability Following Nipple-Sparing Mastectomy. Ann Surg Oncol 2012;19:3171-6. [Crossref] [PubMed]
- Chidester JR, Ray AO, Lum SS, et al. Revisiting the Free Nipple Graft: An Opportunity for Nipple Sparing Mastectomy in Women with Breast Ptosis. Ann Surg Oncol 2013;20:3350. [Crossref] [PubMed]
- Verheyden CN. Nipple-Sparing Total Mastectomy of Large Breasts: The Role of Tissue Expansion. Plast Reconstr Surg 1998;101:1494-500. [Crossref] [PubMed]
- Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg 1976;3:193-203. [PubMed]
- Cho JW, Yoon E, You H, et al. Nipple-Areola Complex Necrosis after Nipple-Sparing Mastectomy with Immediate Autologous Breast Reconstruction. Arch Plast Surg 2015;42:601-7. [Crossref] [PubMed]