Nipple-sparing mastectomy in women at high risk of developing breast cancer
Introduction
With over 1.4 million new cases worldwide per year, breast cancer is the most common malignancy in women and comprises 23% of all female cancers. In the United Kingdom, the age-standardised incidence and mortality are among the highest in the world, and the disease is the commonest cause of death among women aged 40–50. There are multiple risk factors that are known to increase the rate of breast cancer, including age, certain geographical variation, exposure to hormones (age at menarche, first pregnancy and menopause and use of exogenous hormones) and, of course, genetic predisposition.
Whilst there are promising signs for reducing breast cancer risk using hormonal manipulation (1-3), the level of risk reduction falls far short of that provided by surgical removal of breast tissue and does not address the risk of hormone receptor negative breast cancer. Until another reliable risk-reducing measure is developed, bilateral mastectomy will remain a mainstay of management of women at very high risk who want to substantially reduce their chances of developing breast cancer.
The definition of a high risk patient
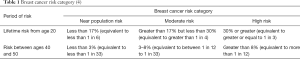
The UK National Institute for Health and Clinical Excellence produces guidelines illustrating thresholds of risk (4) (Table 1).
Identification of high-risk women by family history
Many women with a family history are referred to genetics centres for a comprehensive assessment of their risk. This includes a 3-generation family history (pedigree), and consideration of genetic testing. However, only about 4–5% of breast cancer is thought to be due to inheritance of a high-penetrance, autosomal-dominant, cancer-predisposing gene (5,6), the vast majority being caused by the interplay of lower risk genes and environmental factors. Therefore it is important to estimate the risk to identify women most likely to carry a gene mutation, to offer them genetic testing so that those at very high risk can take steps to modify their risk if they so wish.
In Europe, risk estimation is based mainly on the Claus dataset, which was developed prior to the discovery of the BRCA genes (5-8). In the USA, the Gail model of risk estimation is widely used, encompassing a range of patient factors including prior benign breast biopsies (9). As well as these datasets allowing estimation of risk, there are specific computer programs available, including Tyrer-Cuzick (10), BOADICEA (11) and BRCAPRO (12). Of these, only BOADICEA considers polygenic risk, with the others considering only BRCA1/2 within their breast cancer risk algorithms. The NCCN provides comprehensive guidance on criteria for genetic risk evaluation on their website (13).
Both BRCA1 and 2 increase the risk of ovarian cancer, while BRCA2 is also associated with an increased risk of male breast cancer, prostate cancer, and a slightly increased risk of pancreatic cancer. The important features in a family history suggestive of a gene mutation include the age at onset in the affected relatives, the presence of bilateral breast cancer, and other, related, early-onset tumours such as epithelial ovarian cancer or sarcoma. Additional factors include the presence of a specific tumour type (such as triple negative breast cancer or male breast cancer), or multiple primary cancers, which may be either synchronous or metachronous.
There are few pedigrees in which it is possible to be certain of a dominantly-inherited susceptibility. However, the Breast Cancer Linkage Consortium (BCLC) data suggest that in families with four or more cases of early-onset or bilateral breast cancer, the risk of an unaffected woman inheriting a mutation in a predisposing gene is close to 50%. These studies have estimated that the majority of such families harbour mutations in BRCA1 or BRCA2, especially when male breast cancer or ovarian cancer is present. In breast-only families, the frequency of BRCA1/2 involvement falls to below 50% in four-case families (14). Family and epidemiological studies have demonstrated that approximately 70–85% of BRCA1 and BRCA2 mutation carriers develop breast cancer in their lifetime, although the risk is a little lower for BRCA2 (14-17). The very low figures published on small numbers of families from population studies have now been addressed by a meta-analysis (15), which gives risks to 70 years of age of around 70% for BRCA1 and 55% for BRCA2. Given that the BRCA2 risk continues to increase to age 80, a lifetime risk of 70% for both may be more accurate.
Genetic testing—BRCA1, BRCA2 and TP53
Most genetic testing at present concentrates on BRCA1 and BRCA2 and in the UK National Health Service is limited to women thought to be at a 10% risk of carrying a mutated gene based either on their family history (4) or on their own cancer history. However, the accuracy of family history-based risk-estimations is compromised in women with a very small family (few siblings, aunts, uncles or cousins) or who lose touch with one side of the family, or both, e.g., if adopted or if the paternal line is uncertain. In response to these limitations, there has been a move towards testing women with cancer who fit certain criteria, as this approach has been fruitful in ovarian cancer (18). Although the published overall detection rate for mutations in isolated breast cancer cases even at very young age may be less than 10% (19), women under the age of 40 years of age with a grade 3 triple-negative cancer have a great than 10% chance of a BRCA1 mutation (20). Implementation of simplified criteria for testing in a UK setting (without reference to family history) has resulted in rates of 12.1% in a woman under the age of 40 with any phenotype of breast cancer, 13.6% in any woman with bilateral breast cancer where both occurred under the age of 60 and 10.2% in any woman with triple negative breast cancer. The rate of mutations in women who has developed both ovarian and breast cancer was 27.8% and in males with breast cancer, 11.5% (21). Studies have demonstrated that women who know their BRCA status before surgery are more likely to opt for bilateral mastectomy (i.e., contralateral risk-reducing mastectomy) than those who do not have this information (22,23). Moreover, the presence of a mutation allows predictive testing of relatives, hence identifying other family members at high risk.
While a number of other genes predispose to a moderately increased risk of breast cancer, there is limited clinical utility shown for the testing of genes other than BRCA1 and 2, and rarely TP53 in the absence of other syndromic features. The TP53 gene on chromosome 17p predisposes to early breast cancer, and the risk of developing breast cancer aged below 30 is higher than that for BRCA1. These patients are at a substantially increased risk of other tumours including adrenocortical tumours, brain malignancy and sarcoma (Li-Fraumeni Syndrome). Other genes associated with the development of breast cancer are listed below, but few reach a risk level to warrant risk-reducing surgery. Of note, an increased risk of bilateral breast cancer has only been clearly demonstrated in those with germline mutations in the BRCA1, BRCA2, TP53, CDH1 and STK11, with no clear evidence for a significantly increased risk of contralateral breast cancer in those with mutations in other genes.
Women with a germline mutation in TP53, the gene associated with Li-Fraumeni syndrome have lifetime risks of any cancer in excess of 90% (24) and breast cancer specific risks over 50% (25). Many will develop multiple malignancies over their lifetime, with the risk beginning in early childhood. The breast cancer risk increases in the third decade, with up to a third of women diagnosed before the age of 30. As a result, many centres offer routine TP53 testing to all women diagnosed with breast cancer <30 years, with a reported detection rate of 5% (19).
Currently, guidelines recommend high-risk breast screening with MRI from the age of 20–25 in carriers; and consideration of risk-reducing mastectomy although direct evidence for the latter is lacking. TP53 mutations also sensitize carriers to ionizing radiation, with increased rates of chest wall sarcoma reported in those who have undergone breast-conserving surgery and adjuvant radiotherapy. For this reason, therapeutic mastectomy is the treatment of choice where possible in those diagnosed with unilateral breast cancer.
Other breast cancer susceptibility genes
Cowden’s syndrome is caused by germline mutations in the PTEN gene, with carriers at increased risk of breast cancer, in addition to thyroid, endometrial and GI malignancies, and benign breast disease (26). Although rare in the general population, women who carry a mutation in PTEN have lifetime breast cancer risks of 25–50%, with most diagnosed pre-menopausally (27,28). Management guidelines vary, but most are consistent with the NCCN guidelines (13) that recommend mammography +/− MRI from the age of 30 and consideration of risk-reducing mastectomy, although trial evidence to support this is lacking.
Germline mutations in CDH1 are associated with hereditary diffuse gastric cancer, but also predispose women to developing lobular breast cancer, with a lifetime risk of around 40%. The increased risk appears to begin before the age of 30, and also predisposes to bilateral malignancy (29,30). As with TP53 and PTEN, there is no direct evidence to support the use of RRM, but it is recommended that this should be considered on a case-by-case basis in unaffected women with a family history of breast cancer (13).
The role of germline mutations in PALB2 in breast cancer risk has become better defined in recent years. The risk to carriers of a PALB2 mutation appears to vary by population and family history of breast cancer, but female carriers are estimated to have lifetime breast cancer risks of between 23–91% by age 75, with the largest study to date suggesting a risk of 33–58% (31-33). It should be noted that several studies have tested only for recurrent mutations, which may skew the data. PALB2 mutations have also been associated with a two-fold increase in the risk of ovarian cancer, and an eight-fold increase in the risk of male breast cancer (31). It appears that PALB2 mutations may also be associated with a higher risk of triple negative breast cancer, however this is based on small numbers of patients and should be interpreted with caution. The wide cancer estimates make general recommendations difficult, but most recommend high-risk breast screening and consideration of risk-reducing mastectomy, especially in those with a family history.
Several variants in CHEK2 have been associated with an increased risk of breast cancer, of which the most strongly associated is the 1100delC mutation (34). As with many of the moderate penetrance genes, the breast cancer risk for carriers varies significantly between carriers identified from unselected breast cancer series, and by those detected from series selected for a strong family history of breast cancer (35-37). The largest meta-analysis to date suggests lifetime risks by age 80 of 20% for oestrogen receptor positive (ER +ve) breast cancer, and 3% for oestrogen receptor negative breast cancer, putting carriers below the threshold for risk-reducing mastectomy commonly used in the UK (36). Carriers are generally offered moderate risk breast screening, but not risk-reducing mastectomy unless there is a strong family history. A few cases of homozygous 1100delC carriers have been reported, with significantly higher lifetime risks than heterozygous carriers (38). These women should be considered for high risk breast screening and it may be reasonable to consider surgical risk-reduction. However these women are rare, even in populations with higher rates of 1100delC mutations.
Protein-truncating mutations in ATM have been associated with a moderately increased risk of breast cancer, with heterozygous carriers estimated to have twice the population risk of breast cancer (39,40). There is also evidence that a small number of missense mutations may confer a similar risk, although most missense mutations have not been clearly associated with increased risk (40). Currently, moderate risk breast screening is recommended for affected women, but risk-reducing mastectomy is not routinely suggested for heterozygous carriers.
In addition to the moderate and high risk genes, a number of single nucleotide polymorphisms (SNPs) that increase breast cancer risk have been identified from genome-wide association studies (GWAS). These SNPs, while common in the general population are only associated with a very small increased risk of breast cancer by themselves, such as a RR of 1.1 or 1.2. However, as they are common, it is possible for an individual to inherit several. There is significant interest currently in using population-based SNP profiling for breast cancer to estimate an individual’s future risk, with breast screening either intensified or reduced as a result (41).
Other risk factors
Several long term studies of so-called “high risk” lesions have reported varying levels of absolute risk according to histopathological findings in benign breast biopsies (42-44). While higher risk lesions such as multifocal atypical hyperplasia may be associated with a risk approaching 50% at 25 years (43), it must be remembered that this is only found in 15% of women with atypical hyperplasia, and atypical hyperplasia itself represents just 5–8% of benign breast biopsies. Thus this level of risk is reached in less than 1% of women with benign breast disease. Both the Partners (42) and the Memorial Sloan Kettering (44) series demonstrated an absolute risk of 2% per year or more for LCIS. Age and family history did not modify the risk but “chemo-preventative” strategies (i.e., endocrine therapy, excluding surgery) resulted in a 65–70% relative reduction in that risk. Thus surgery is rarely warranted for women with so-called high-risk lesions.
Similarly, while increasing risk overall, environmental exposures have a limited impact on future risk of breast cancer in most individuals. One exception is mantle radiotherapy for Hodgkin’s disease conferring a 12.2% risk at 25 years follow up (45). Other risk factors such as the use of hormone replacement therapy have their effect on breast cancer incidence at a population level. For example, 10 years’ use of HRT is estimated to result in 5 [95% confidence interval (CI): 3–7] additional breast cancers per 1,000 users of oestrogen-only preparations and 19 (95% CI: 15–23) additional cancers per 1,000 users of oestrogen-progestogen combinations such that surgery would not be considered (46). Modifiable lifestyle risk factors such as obesity and alcohol intake should be highlighted to women concerned about their risk of breast cancer.
Risk-reducing surgery
In the UK, the NICE guidelines (4) advise that bilateral risk-reducing mastectomy is appropriate only for a small proportion of women who are from high-risk families or have a pathogenic gene mutation. They should be managed by a multidisciplinary team. Bilateral mastectomy should be raised as a risk-reducing strategy option with all women at high risk, but chemoprevention and surveillance strategies should also be discussed and the additional risk factors such as age should be taken into account. Women considering bilateral risk-reducing mastectomy should have genetic counseling in a specialist cancer genetic clinic before a decision is made. Women should be counseled pre-operatively about psychosocial and sexual consequences of bilateral risk-reducing mastectomy, including being offered access to support groups and women who have undergone the procedure, and they should be warned of the possibility of breast cancer being diagnosed histologically. They should be able to discuss their breast reconstruction options (immediate and delayed) with a member of a surgical team with specialist oncoplastic or breast reconstructive skills and such a surgeon should carry out risk-reducing mastectomy and/or reconstruction (4).
The seminal retrospective study by Hartmann et al. (47) examined the incidence of, and modelled the risk of death from, breast cancer after a median follow-up of 14 years among 639 women thought to be at high or moderate risk as a result of their a family history of breast or ovarian cancer. Five hundred and seventy five had undergone bilateral “subcutaneous” and 64 bilateral “total” prophylactic mastectomy. This showed a reduction in risk of developing breast cancer of 90–94% in high risk women and 89.5% in women at moderate risk. There was no statistically significant difference in incidence according to type of mastectomy, with seven developing breast cancer in the subcutaneous group and none in the total mastectomy group. One of the women developed a breast cancer in the nipple 6 years after surgery. A subsequent subgroup analysis of BRCA1 or 2 gene mutation carriers (48), reported no breast cancers developing by 13.4 years in the group which had undergone bilateral risk-reducing mastectomy, though there were only 18 women. Later studies e.g., Rebbeck et al. (49) all show a reduction in the incidence of breast cancer among women who undergo bilateral mastectomy, though the follow up is too short to consider whether this translates to a survival difference. An exception is Ingham et al. (50) who reported on a large series of 346 BRCA1 carriers and 345 BRCA2 carriers with 10 years of follow up. Some had undergone risk-reducing surgery (bilateral mastectomy, salpingo-oophorectomy or both). Cancer developed in 105 and 122 women in the BRCA1 and 2 groups respectively and the hazard of death was lower following risk-reducing surgery (P<0.001). For BSO (adjusted for age) this was 0.22 (95% CI: 0.08–0.61). For bilateral mastectomy it was 0.25 (0.03–1.81) and for both it was 0.14 (0.02–1.02). In fact, only 58 women had undergone bilateral mastectomy only, and the small sample size will have contributed to the wide CIs. In addition, risk-reducing mastectomy is usually carried out at a young age, when the hazard of death would be low anyway. By contrast, in this study and elsewhere, risk-reducing bilateral salpingo-oophorectomy reduces ovarian and breast cancer risks and this has translated to improvements in survival (51). The impact of age at the time of surgery on risk reduction warrants further discussion. Giannakeas and Narod (52) modelled this for a woman with a BRCA mutation but assumed intact ovaries, such that the impact of death from ovarian cancer limited the impact of bilateral mastectomy on mortality considerably. Nonetheless, the effect of age at the time of mastectomy was striking and this should be emphasized to women considering this surgery. For example, they report that a 25-year-old undergoing mastectomy reduces her risk of breast cancer to the age of 80 by 64% and this translates into a reduction in mortality of 14.7% (not more because the likelihood of dying of ovarian cancer or other causes later in life is much greater). For a woman of 60, the figures fall to 19.2% and 2.9% respectively. The impact of bilateral mastectomy after risk-reducing oophorectomy is hard to model, because of the effect of oophorectomy on breast cancer risk, but removal of the greatest risk of competing mortality (ovarian cancer) is likely to increase the impact of minimizing the breast cancer risk by surgery. Giannakeas and Narod concede that women in their 50s and 60s often undergo risk-reducing surgery for cancer prevention, rather than to reduce their risk of mortality.
The uptake of risk-reducing mastectomy varies by county, and several studies have reported on this, see Table 2. With increasing availability of high-quality, safe immediate breast reconstruction, and recent publicity, most notably by the celebrity Angelina Jolie, the current rates of uptake are likely to be higher. Countries without a nationalized health service and in which risk-reducing surgery is not covered by insurance such as Japan (61), have lower rates, with the first risk-reducing mastectomy for a Japanese BRCA mutation carrier reported in 2016 (62). Nonetheless there must also be cultural differences to explain the very low rates in France.

Full table
Some groups have investigated the reasons for proceeding with risk-reducing surgery. Singh et al. (55) showed that having a first or second degree relative who died from breast cancer was a strong predictor for uptake (OR 11.0, P=0.005) and Haroun et al. (63) showed relatives with breast cancer and fear of cancer were associated with an increased likelihood of opting for surgery.
Nipple-sparing mastectomy in high-risk patients
Women at high risk who are considering surgical options for breast cancer risk-reduction often know more about the reconstructive options than the choices around how to do a mastectomy. It is important to describe the spectrum from simple mastectomy, through skin-sparing to nipple-sparing (or total skin-sparing) mastectomy. It is also crucial to inform the woman that risk-reducing surgery does not reduce her risk of breast cancer to zero. Histological studies invariably show residual breast tissue (64,65) even in cadaveric studies in which very thin skin flaps were fashioned (66) suggesting that complete removal of all breast tissue is an unattainable goal (67), though given the increasing risk with increasing thickness of skin flaps (65) surgeons must avoid the temptation to leave excessively thick flaps in the interests of better cosmesis. While histological studies have also shown terminal ductal lobular units (TDLUs) in the nipple (68-70), the surface area of epithelium retained in the nipple is likely to be substantially less than under the skin flaps (71). In a recent series of 150 patients (298 breasts) undergoing NSM for risk-reduction, occult disease was found in only 4 patients (2.7% of which 2 were in situ and one invasive disease). No cases contained disease in the nipple (72). Other series have demonstrated occult disease in the retro-areolar or nipple specimens, mostly in women with occult disease within the breast (73,74). These cases highlight the need for careful pre-operative imaging and histological evaluation of the specimen, even in the risk-reducing setting.
The studies cited earlier summarize the reduction in risk of breast cancer from bilateral mastectomy. At the time when BRCA1 and BRCA2 were discovered in the 1990s, concern persisted over the safety of preservation of the nipple. Initial case series were reassuring (though with short follow up). Table 3 highlights series of risk-reducing nipple-sparing mastectomy with at least 2 years of follow-up. Even this duration of follow-up in the prophylactic setting, is unlikely to be truly reflective of the level of risk-reduction. Other series were not included if, for example, it was not possible to discern whether results related to NSM (rather than SSM) or therapeutic (rather than risk-reducing) cases.

Full table
A systematic review written in French (82) reported on 3,716 prophylactic nipple-sparing mastectomies in 19 articles. The average follow up was 38.4 months and 29.4% of patients had a BRCA1 or BRCA2 mutation. The average rate of development of cancer within the nipple-areola complex was 0.004% and other local presentation 0.2%.
The American Society of Breast Surgery NSM registry represents one of the largest series of nipple-sparing mastectomies (83) and will be described in detail in another article in this issue. Of relevance here, 1,252 NSMs carried out for risk-reduction had an incidence of breast cancer of 0.2% after a median follow up of 23.3 months. Although duration of follow up was not broken down according to indication for NSM (therapeutic or risk-reducing), since the cautious re-introduction of NSM tended to be in the risk-reducing setting before therapeutic, there is reason to believe that the follow up after risk-reducing NSM is at least this long.
An additional point to consider is the fact that the risk of developing cancer in the nipple is likely to be proportional to the risk of developing cancer. As described above, proven BRCA mutation carriers have higher risk than those with a family history, or “high-risk” lesions. In many series, the BRCA-status of many of the women undergoing “risk-reducing” mastectomy was negative or unknown, hence the risk level in these case series is unknown. Series reporting specifically on NSM in BRCA patients are limited. Recently, Jakub et al. (84) reported on 346 patients with BRCA mutations undergoing risk-reducing NSM (either bilateral, or with a contralateral therapeutic mastectomy). After a median of 34 months’ follow up, none had developed breast cancer. Although this follow up is relative short in a young population, the authors performed risk estimates which suggested that 22 women would have developed breast cancer in that time. The impact of concurrent bilateral salpingo-oophorectomy was not assessed.
It is also reassuring to note that the evidence amassing for nipple-sparing mastectomy in patients with breast cancer suggests that with careful patient selection, preservation of the nipple does not confer an excess risk. As an example, a series published in July 2017 of 2,182 patients with cancer undergoing nipple-sparing mastectomy over a ten-year period reported that no patients have had a recurrence involving the retained nipple-areola complex (85). The overall follow up was not reported, as the article focused on the long-term follow up of the cases to 2012.
A key question, as highlighted by Jensen (86) in a letter in response to Smith et al. (85), is whether a recurrence (or de novo development of cancer in the nipple) has a significant impact. Data on this issue are sparse and the best information is extrapolated from cases in the therapeutic mastectomy literature in which the incidence is much greater. Most notably, Petit et al. reported on 934 cases of NSM with a median follow up of 50 months (87). There were 11 local recurrences in the nipple, seven presenting as Paget’s disease, associated with DCIS in the underlying ducts and four with invasive carcinoma. The recurrences were removed and the patients were all disease-free after a median of 33 months. It is crucial, therefore, that patients are reminded that risk-reducing surgery does not reduce their risk to zero, and that they know they should report any nodule in or under the skin and any change in the preserved nipple.
Satisfaction after risk-reducing mastectomy
Early studies of women who had undergone bilateral risk-reducing surgery confirmed that this led to a reduction in concern about breast cancer risk (47,88-91), though with negative consequences in terms of physical appearance. It is this area which is most likely to be affected by the subsequent improvements in reconstructive options, including preservation of the nipple. The Mastectomy Reconstruction Outcomes Consortium has recently published on health-related quality of life in 204 women who have undergone risk-reducing bilateral mastectomy. Anxiety was significantly lower and psychosocial well-being was significantly higher at both 1 and 2 years (P<0.01), and physical well-being of the chest and upper body was significantly worse at 1 year (P<0.01). Somewhat surprisingly, satisfaction with the breasts was significantly higher at both 1 and 2 years compared to baseline (92). While this is the patient’s subjective reporting and may reflect her calibration against expectation, it is nonetheless also testament to the excellent results currently achievable with immediate reconstruction.
Impact of nipple-sparing mastectomy on risk-reduction in the high-risk population
The expectation of an aesthetically acceptable result from mastectomy and reconstruction is likely to lead women to consider risk-reduction surgery more favourably. Metcalfe et al. (93) carried out a modelling study which predicted that if the uptake of surgery by unaffected BRCA carriers was 20% if reconstruction was not available, and 50% if “subcutaneous mastectomy” were offered, and if subcutaneous mastectomy reduces the risk of disease by 95%, being offered subcutaneous mastectomy would reduce the incidence of breast cancer from 320 to 215 per 1,000 BRCA mutation carriers. In 2010, 32% of unaffected BRCA carriers in our unit had proceeded to surgery (59). As mentioned above, rates of uptake are likely to have increased as a result of recent publicity (94), but also the availability of cosmetically acceptable reconstructive surgery. Thus, far from being a frivolous consideration compromising risk-reduction, sparing the nipple may be contributing to greater uptake of risk-reducing surgery in a cohort at significant risk.
Acknowledgements
The Royal Marsden/Institute for Cancer Research is a National Institute for Health Research Biomedical Research Centre. This support is acknowledged.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005;97:1652-62. [Crossref] [PubMed]
- Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst 2007;99:272-82. [Crossref] [PubMed]
- Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696-706. [Crossref] [PubMed]
- National Institute for Health and Clinical Excellence. Familial breast cancer: Classification and care of people at risk of familial breast cancer and management of breast cancer and related risks in people with a family history of breast cancer 2013 [updated March 2017]. Available online: https://www.nice.org.uk/guidance/cg164/evidence/full-guideline-pdf-190130941
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994;73:643-51. [Crossref] [PubMed]
- Newman B, Austin MA, Lee M, et al. Inheritance of human breast cancer: evidence for autosomal dominant transmission in high-risk families. Proc Natl Acad Sci U S A 1988;85:3044-8. [Crossref] [PubMed]
- Evans DG, Lalloo F. Risk assessment and management of high risk familial breast cancer. J Med Genet 2002;39:865-71. [Crossref] [PubMed]
- Vasen HF, Haites NE, Evans DG, et al. Current policies for surveillance and management in women at risk of breast and ovarian cancer: a survey among 16 European family cancer clinics. European Familial Breast Cancer Collaborative Group. Eur J Cancer 1998;34:1922-6. [Crossref] [PubMed]
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879-86. [Crossref] [PubMed]
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23:1111-30. [Crossref] [PubMed]
- Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 2004;91:1580-90. [Crossref] [PubMed]
- Berry DA, Iversen ES Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol 2002;20:2701-12. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian 2017 [updated 3rd October 2017]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62:676-89. [Crossref] [PubMed]
- Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117-30. [Crossref] [PubMed]
- Evans DG, Shenton A, Woodward E, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 2008;8:155. [Crossref] [PubMed]
- Ford D, Easton DF, Bishop DT, et al. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994;343:692-5. [Crossref] [PubMed]
- George A, Riddell D, Seal S, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep 2016;6:29506. [Crossref] [PubMed]
- Evans DG, Moran A, Hartley R, et al. Long-term outcomes of breast cancer in women aged 30 years or younger, based on family history, pathology and BRCA1/BRCA2/TP53 status. Br J Cancer 2010;102:1091-8. [Crossref] [PubMed]
- Robertson L, Hanson H, Seal S, et al. BRCA1 testing should be offered to individuals with triple-negative breast cancer diagnosed below 50 years. Br J Cancer 2012;106:1234-8. [Crossref] [PubMed]
- Kemp ZaRN. Identifying genetic risk in the prevalent breast cancer population. UK Interdisciplinary Breast Cancer Symposium; Manchester, UK. 2018.
- Chiba A, Hoskin TL, Hallberg EJ, et al. Impact that Timing of Genetic Mutation Diagnosis has on Surgical Decision Making and Outcome for BRCA1/BRCA2 Mutation Carriers with Breast Cancer. Ann Surg Oncol 2016;23:3232-8. [Crossref] [PubMed]
- Yadav S, Reeves A, Campian S, et al. Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort analysis. Hered Cancer Clin Pract 2017;15:11. [Crossref] [PubMed]
- Chompret A, Brugieres L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000;82:1932-7. [PubMed]
- Mai PL, Best AF, Peters JA, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016;122:3673-81. [Crossref] [PubMed]
- Brownstein MH, Wolf M, et al. Cowden's disease: a cutaneous marker of breast cancer. Cancer 1978;41:2393-8. [Crossref] [PubMed]
- Bubien V, Bonnet F, Brouste V, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013;50:255-63. [Crossref] [PubMed]
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012;18:400-7. [Crossref] [PubMed]
- Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007;297:2360-72. [Crossref] [PubMed]
- Pharoah PD, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001;121:1348-53. [Crossref] [PubMed]
- Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med 2014;371:497-506. [Crossref] [PubMed]
- Cybulski C, Kluzniak W, Huzarski T, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol 2015;16:638-44. [Crossref] [PubMed]
- Southey MC, Goldgar DE, Winqvist R, et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet 2016;53:800-11. [Crossref] [PubMed]
- Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 2002;31:55-9. [Crossref] [PubMed]
- Kuusisto KM, Bebel A, Vihinen M, et al. Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res 2011;13:R20. [Crossref] [PubMed]
- Schmidt MK, Hogervorst F, van Hien R, et al. Age- and Tumor Subtype-Specific Breast Cancer Risk Estimates for CHEK2*1100delC Carriers. J Clin Oncol 2016;34:2750-60. [Crossref] [PubMed]
- Krivokuca A, Dobricic J, Brankovic-Magic M. CHEK2 1100delC and Del5395bp mutations in BRCA-negative individuals from Serbian hereditary breast and ovarian cancer families. J BUON 2013;18:594-600. [PubMed]
- Adank MA, Jonker MA, Kluijt I, et al. CHEK2*1100delC homozygosity is associated with a high breast cancer risk in women. J Med Genet 2011;48:860-3. [Crossref] [PubMed]
- Decker B, Allen J, Luccarini C, et al. Rare, protein-truncating variants in ATM, CHEK2 and PALB2, but not XRCC2, are associated with increased breast cancer risks. J Med Genet 2017;54:732-41. [Crossref] [PubMed]
- Renwick A, Thompson D, Seal S, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 2006;38:873-5. [Crossref] [PubMed]
- Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst 2015;107. [Crossref] [PubMed]
- Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat 2012;136:627-33. [Crossref] [PubMed]
- Hartmann LC, Degnim AC, Santen RJ, et al. Atypical hyperplasia of the breast--risk assessment and management options. N Engl J Med 2015;372:78-89. [Crossref] [PubMed]
- King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol 2015;33:3945-52. [Crossref] [PubMed]
- Taylor AJ, Winter DL, Stiller CA, et al. Risk of breast cancer in female survivors of childhood Hodgkin's disease in Britain: a population-based study. Int J Cancer 2007;120:384-91. [Crossref] [PubMed]
- Beral V. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362:419-27. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst 2001;93:1633-7. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- Ingham SL, Sperrin M, Baildam A, et al. Risk-reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Res Treat 2013;142:611-8. [Crossref] [PubMed]
- Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg 2016;212:660-9. [Crossref] [PubMed]
- Giannakeas V, Narod SA. The expected benefit of preventive mastectomy on breast cancer incidence and mortality in BRCA mutation carriers, by age at mastectomy. Breast Cancer Res Treat 2018;167:263-7. [Crossref] [PubMed]
- Chai X, Friebel TM, Singer CF, et al. Use of risk-reducing surgeries in a prospective cohort of 1,499 BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 2014;148:397-406. [Crossref] [PubMed]
- Garcia C, Wendt J, Lyon L, et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol 2014;132:428-33. [Crossref] [PubMed]
- Singh K, Lester J, Karlan B, et al. Impact of family history on choosing risk-reducing surgery among BRCA mutation carriers. Am J Obstet Gynecol 2013;208:329.e1-6. [Crossref] [PubMed]
- Schwartz MD, Isaacs C, Graves KD, et al. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer 2012;118:510-7. [Crossref] [PubMed]
- Julian-Reynier C, Mancini J, Mouret-Fourme E, et al. Cancer risk management strategies and perceptions of unaffected women 5 years after predictive genetic testing for BRCA1/2 mutations. Eur J Hum Genet 2011;19:500-6. [Crossref] [PubMed]
- Skytte AB, Gerdes AM, Andersen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet 2010;77:342-9. [Crossref] [PubMed]
- Bancroft EK, Locke I, Ardern-Jones A, et al. The carrier clinic: an evaluation of a novel clinic dedicated to the follow-up of BRCA1 and BRCA2 carriers--implications for oncogenetics practice. J Med Genet 2010;47:486-91. [Crossref] [PubMed]
- Evans DG, Lalloo F, Ashcroft L, et al. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol Biomarkers Prev 2009;18:2318-24. [Crossref] [PubMed]
- Yoshimura A, Okumura S, Sawaki M, et al. Feasibility study of contralateral risk-reducing mastectomy with breast reconstruction for breast cancer patients with BRCA mutations in Japan. Breast Cancer 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Maeshima Y, Oseto K, Katsuragi R, et al. Experience with Bilateral Risk-Reducing Mastectomy for an Unaffected BRCA Mutation Carrier. J Breast Cancer 2016;19:218-21. [Crossref] [PubMed]
- Haroun I, Graham T, Poll A, et al. Reasons for risk-reducing mastectomy versus MRI-screening in a cohort of women at high hereditary risk of breast cancer. Breast 2011;20:254-8. [Crossref] [PubMed]
- Cao D, Tsangaris TN, Kouprina N, et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol 2008;15:1330-40. [Crossref] [PubMed]
- Torresan RZ, dos Santos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [Crossref] [PubMed]
- Goldman LD, Goldwyn RM. Some anatomical considerations of subcutaneous mastectomy. Plast Reconstr Surg 1973;51:501-5. [Crossref] [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Stolier AJ, Wang J. Terminal duct lobular units are scarce in the nipple: implications for prophylactic nipple-sparing mastectomy: terminal duct lobular units in the nipple. Ann Surg Oncol 2008;15:438-42. [Crossref] [PubMed]
- Rosen PP, Tench W. Lobules in the nipple. Frequency and significance for breast cancer treatment. Pathol Annu 1985;20:317-22. [PubMed]
- Reynolds C, Davidson JA, Lindor NM, et al. Prophylactic and therapeutic mastectomy in BRCA mutation carriers: can the nipple be preserved? Ann Surg Oncol 2011;18:3102-9. [Crossref] [PubMed]
- Coopey SB, Smith BL. The Nipple is Just Another Margin. Ann Surg Oncol 2015;22:3764-6. [Crossref] [PubMed]
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 2015;22:370-6. [Crossref] [PubMed]
- Garcia-Etienne CA, Borgen PI. Update on the indications for nipple-sparing mastectomy. J Support Oncol 2006;4:225-30. [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [Crossref] [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [Crossref] [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Munhoz AM, Aldrighi CM, Montag E, et al. Clinical outcomes following nipple-areola-sparing mastectomy with immediate implant-based breast reconstruction: a 12-year experience with an analysis of patient and breast-related factors for complications. Breast Cancer Res Treat 2013;140:545-55. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- Peled AW, Irwin CS, Hwang ES, et al. Total skin-sparing mastectomy in BRCA mutation carriers. Ann Surg Oncol 2014;21:37-41. [Crossref] [PubMed]
- Muller T, Baratte A, Bruant-Rodier C, et al. Oncological safety of nipple-sparing prophylactic mastectomy: A review of the literature on 3716 cases. Ann Chir Plast Esthet 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mitchell SD, Beitsch PD. The American Society of Breast Surgeons Nipple-Sparing Mastectomy Registry. In: Harness JK, Willey SC. editors. Operative Approaches to Nipple-Sparing Mastectomy, Indications Techniques and Outcomes. 1st ed. Springer, 2017:235-48.
- Jakub JW, Peled AW, Gray RJ, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg 2018;153:123-9. [Crossref] [PubMed]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Jensen JA. Nipple-Sparing Mastectomy. J Am Coll Surg 2018;226:108. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol 2012;23:2053-8. [Crossref] [PubMed]
- Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol 2008;26:3943-9. [Crossref] [PubMed]
- Frost MH, Schaid DJ, Sellers TA, et al. Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA 2000;284:319-24. [Crossref] [PubMed]
- Hatcher MB, Fallowfield L, A'Hern R. The psychosocial impact of bilateral prophylactic mastectomy: prospective study using questionnaires and semistructured interviews. BMJ 2001;322:76. [Crossref] [PubMed]
- Heiniger L, Butow PN, Coll J, et al. Long-term outcomes of risk-reducing surgery in unaffected women at increased familial risk of breast and/or ovarian cancer. Fam Cancer 2015;14:105-15. [Crossref] [PubMed]
- McCarthy CM, Hamill JB, Kim HM, et al. Impact of Bilateral Prophylactic Mastectomy and Immediate Reconstruction on Health-Related Quality of Life in Women at High Risk for Breast Carcinoma: Results of the Mastectomy Reconstruction Outcomes Consortium Study. Ann Surg Oncol 2017;24:2502-8. [Crossref] [PubMed]
- Metcalfe KA, Semple JL, Narod SA. Time to reconsider subcutaneous mastectomy for breast-cancer prevention? Lancet Oncol 2005;6:431-4. [Crossref] [PubMed]
- Evans DG, Wisely J, Clancy T, et al. Longer term effects of the Angelina Jolie effect: increased risk-reducing mastectomy rates in BRCA carriers and other high-risk women. Breast Cancer Res 2015;17:143. [Crossref] [PubMed]