Nipple sparing mastectomy and direct to implant breast reconstruction, validation of the safe procedure through the use of laser assisted indocyanine green fluorescent angiography
Introduction
During the last decade, nipple-sparing mastectomies (NSMs) have gained acceptance in breast surgical oncology (1).
The overall complication rate of NSM ranges from 0% to 48% (2). Nipple areola complex (NAC) and skin necrosis are the more ominous and the most frequent adverse events. Moyer et al. in 2012 reported 37.5% of partial nipple necrosis (3), reporting perfusion reductions and relative complications in circumareolar/radial incisions, on the contrary good outcomes in vertical or inframammary fold approaches; a superimposable experience is reported by Boneti et al. (4) with a partial or complete necrosis of the NAC ranging from 3.5% to 13% (5,6). Chirappapha and colleagues underscore the risk of skin and NAC necrosis occurring in patients with larger breasts (7). Our experience in a retrospective analysis of 2,023 NSM report a range of 6.1% of flap necrosis considering a miscellanea of surgical incisions, mastectomy flap thickness and some wide inclusion criteria (2).
In order to assess the viability of both mastectomy skin flaps and NAC, several attempts have been made to predict skin flap perfusion, including clinical parameters such as evaluation of skin colour, capillary refill time, dermal edge bleeding and skin temperature, and instrumental diagnosis by using hand-held Doppler, laser Doppler flowmetry, and fluorescein angiography (8).
In recent years, intraoperative laser-assisted indocyanine green (ICG) fluorescent angiography (LA-ICGA) has been proposed by several authors in order to provide a real-time visualization of post-mastectomy skin flap perfusion, due to the important features of the fluorescent agent ICG in terms of short half-life, strong binding to plasma proteins, excellent safety profile and rapid clearance (9).
Our purpose, in the present study, is to evaluate a safe surgical technique in NSM and direct to implant (DTI) breast reconstruction through intraoperative LA-ICGA. From January 2015 to September 2015 we have adopted in our patients a series a safe procedure to avoid inadequate skin flap perfusion concerning properly surgical technique and exclusion criteria.
We report our retrospective experience corroborated by intraoperative LA-ICGA to demonstrate the safety and reliability of the oncoplastic procedure proposed. We also advocate the central role of oncoplastic surgeon as “one-man band” to manage breast cancer from the oncological procedure to the breast reconstruction.
Methods
A retrospective clinical study was performed from January 2015 to September 2015. All patients presented at our Institution with breast cancer or BRCA 1–2 genetic mutations and high family risk. The study group consisted of 40 consecutive patients undergoing a monolateral/bilateral NSM and DTI breast reconstruction with intraoperative use of LA-ICGA.
Patient selection
The patients enrolled were divided into two groups depending on breast volume and weight of mastectomy: group A, 20 patients with small breast; group B, 20 patients with medium breast. The cut-off value between small and medium breast was up to 300 gr weight of mastectomies for group A and up to 450 gr weight of mastectomy for group B.
At our Institution, ethics approval was not required considering the well renowned intraoperative LA-ICGA technique and the oncoplastic procedure described, the study was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). A proper written informed consent was obtained from all the patients.
Exclusion criteria were: body mass index greater than 30 kg/m2 and large breasts, age older than 65 years, smokers, a history of neoadjuvant chemotherapies and/or radiation therapy.
Surgical procedure
All operations were performed under general anaesthesia by the Senior Author with patients in semi seated positions. During mastectomy, the patient’s upper limbs were abducted to allow axillary access, and then adducted to relax the pectoralis major muscle for breast reconstruction. Lateral inframammary fold surgical incisions were used in accord with the study design such as scissors was used solely for mastectomy. A separate axillary incision was performed for the sentinel lymph node biopsy or axillary lymph node dissection (ALND) when needed.
Intraoperative histologic analysis of sections from sub-nipple-areola complex tissue was routinely performed. The breast tissue was removed, weighed, and then transported to the pathologist. After mastectomy, skin-flap thickness was measured by means of graduated callipers at upper outer, lower outer, upper inner, lower inner and beneath the NAC, and the average was between 5 and 8 mm. In all patients, reconstructive implant procedures always began immediately with definitive anatomical breast implant Mentor CPG Cohesive IIITM (Mentor Corp., Santa Barbara, USA); all the implants have been chosen according to preoperative breast shape, mastectomy weight, width of the breast and respecting the contralateral side shape in monolateral procedure. Reconstructive procedure in group A was DTI without any kind of additional device: the implant was covered by pectoralis major muscle (with the costal origin of the pectoralis major divided at all long the inframammary fold) and the anterior serratus muscle for the lateral part of the implant. In medium breasts (group B), the surgical pocket was performed similarly to the group A by a sub muscular dissection of the pectoralis major muscle plus SurgiMend PRS (TEI, Biosciences, Inc., Boston, MA, USA); so the implant was inserted in a sub-muscular layer superiorly, the inferior and lateral part of the implant was covered by acellular bovine dermal collagen matrix sling.
A drain was always placed in the mastectomy pocket and another one in the axilla when ALND was performed.
Intraoperative LA-ICGA
Post-mastectomy skin flap perfusion was evaluated by using Quest SpectrumTM (Quest Medical Imaging, Akron, Ohio, USA) Fluorescence Imaging Platform. A single dose of ICG dye (0.2 mg/kg) was administered intravenously followed by 10 cc of saline flush. Fluorescence images were captured using the digital camera on the Quest SpectrumTM that is usually positioned at a distance of about 20 cm from the operative field. All images are then managed and elaborated by the Spectrum Capture Suite program that allows a real-time visualization of the fluorescence signals and record the video data. The perfusion analysis was obtained by using Quest SpectrumTM before and after breast implant placement, in order to detect any difference of perfusion and viability caused by the presence of the implant.
Postoperative care
Pharmacologic therapy consisting of venous thrombosis prophylaxis was administered 24 hours before surgery and continued for 15 days or until rehabilitation was completed. Broad spectrum antibiotics were administered at anaesthesia induction and continued until drains were removed. Moderate compression was applied with elastic gauze for 2 days after surgery. A bra could usually be worn after postoperative day 10. Standard strictly patient follow-up included the first 21 days postoperative visits (discharge, 3–6–14–21 days) followed by visits at 2, 6 and 12 months, the last follow up visit was at 24 months postop.
Results
Between January 2015 and September 2015, a total of 40 patients divided into two groups qualified for NSM and DTI breast reconstruction. Because of cancerous invasion of the NAC, shown by intraoperative frozen section, 2 patients (group A: 1 patient, group B: 1 patient) were successively excluded from the study, all instead undergoing skin sparing mastectomy. Our cohort then included 38 patients with a mean age of 39 years (range, 22 to 65 years). Mean body mass index was 25 kg/m2 (range, 18 to 30 kg/m2), and body mass index in 4 patients (10.5%) exceeded 27 kg/m2. Overall, 44 NSM were performed, including bilateral procedures in 6 patients (15.7%). All the bilateral procedures were performed for cancer risk reduction, the patients carried the BRCA gene mutation. Table 1 report patient distribution and number of NSM in each group according to tumour dimension.

Full table
Intraoperative sentinel node biopsy was otherwise performed in 31 patients (monolateral procedures). Results of sentinel node biopsy were negative in 28 instances (90%), with discovery of neoplastic microinfiltration or macrometastasis in 3 patients that required further ALND.
DTI breast reconstruction procedures were performed in all the NSM, all the anatomical implants used were CPG Cohesive IIITM (Mentor Corp., Santa Barbara, USA); 19 patients of group A were totally submuscular; 19 patients (group B) were assisted by SurgiMend PRS (TEI, Biosciences, Inc.) for inferior/lateral part of the implant coverage. Mastectomy weight and definitive implant size for each group is reported in Table 2.

Full table
The intraoperative LA-ICGA perfusion analysis before and after breast implant placement demonstrated in all the cases a good flow and perfusion of the flaps and NAC. In all the cases we have not reported any areas of low fluorescence indicating limited flap perfusion.
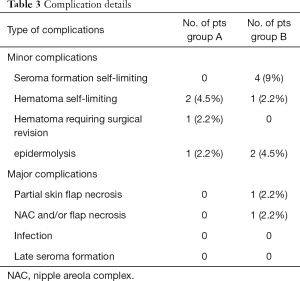
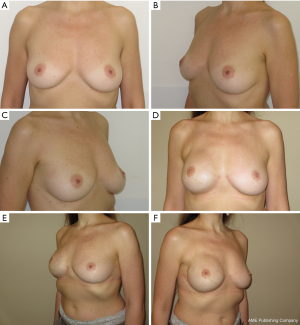
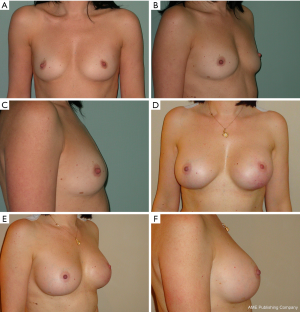
We have divided complications in minor complications that potentially not contemplate breast reconstruction implant failure and major complications that potentially contemplate breast reconstruction implant failure; the encouraging results after 24 months of follow up are showed in Table 3. Regarding the case of partial skin flap necrosis in group B, a locoregional flap was used with implant salvage for a necrosis area less than 3 cm located in the lower pole of the breast. In group B, regarding the unique case of necrosis with a flap area greater than 5 cm including NAC and subsequent implant exposure, a latissimus dorsi flap with implant substitution as a salvage procedure was used. We do not report breast cancer recurrences for all the patients at the end of 24 months follow up. Representative patient photographs are depicted in Figures 1,2.
Full table
Discussion
Since the initial description by Freeman (10,11) in the 1960s, use of NSM has increased substantially (12-14). The oncological safety of NSM is well reported in literature, on the other hand, it is difficult to compare the results due to the difference of patient inclusion/exclusion criteria and different operative methods (15). Moreover, in our recent study of 2,023 NSM procedures, we indicate that the incidence of local recurrences is really low with 22 instances (1.3%). In our opinion, the broad range above reflects extremes of variability in operative skill when dissecting beneath the nipple-areola complex (2,16). We routinely remove all tissue beneath the nipple, whereas many research groups leave variable amounts to augment blood supplied to the nipple-areola complex preventing ominous NAC necrosis (17,18). As demonstrated by Rusby et al., the greater portion of nipple vascularization is peripheral to the nipple, and thus “coring” the nipple should still allow adequate blood supply (19).
Nevertheless, NAC/skin flap necrosis and subsequent implant loss remain the main drawback and a fearsome complication of this technique with reported rates ranging up to 37.5% (20). In the last decade, intraoperative LA-ICGA has been proposed by several authors as an alternative and useful tool to reduce the rates of skin flap necrosis after subcutaneous mastectomy (8,21,22). Recent investigations by Rinker showed that the use of LA-ICGA was associated with an increased accuracy in predicting subcutaneous mastectomy flap necrosis than clinical judgment alone (23). A retrospective analysis by Newman et al. demonstrated a 95% correlation between intraoperative imaging and clinical outcome with 91% specificity and 100% sensitivity (24). We have adopted for the present study intraoperative LA-ICGA to evaluate our methodology for a safe NSM procedure and DTI breast reconstruction. Although LA-ICGA intraoperatively assisted us to identify a good perfusion values and flaps viability in all cases, we have reported in a postop period two patients (4.5%) of skin flap necrosis with one patient (2.2%) of implant failure. Considering the high sensitivity of the LA-ICGA, the properly surgical technique and strictly exclusion criteria adopted, we cannot explain, at this time, what could have happened to the postop flap viability.
Took note of a low rate of minor complications and a 2.2% of implant failure caused by a unique case of full thickness NAC-flap necrosis; we report our encouraging results allowing us to declare how to conduct a safe NSM and DTI breast reconstruction in small and medium breasts reducing the most feared complication: the flap/NAC necrosis and subsequent implant failure. In order to prevent ischemic flaps and subsequent necrosis we have adopted always lateral inframammary fold incision and scissors solely for mastectomy, a mastectomy flap thickness between 5 and 8 mm and properly exclusion criteria.
Skin incision is the most often investigated feature of NSM, undoubtedly because of blood supply to the nipple areola complex and flap viability. Vertical incisions, lazy-S, periareolar or periareolar laterally extended incisions are proposed in literature such as lateral inframammary fold incision (12,25). Our previous results described in literature do suggest that skin incision impacts on complications rate considerably; a significant correlation between incision and aesthetic result was also observed (2).
We have found that inframammary fold incisions enable perfect contouring, with a well-hidden scar without reducing vasculature to the NAC and skin flap; so for our patients series, in the present study, we have used lateral inframammary fold incision to guarantee skin flap and NAC viability.
We report a solely use of scissors for mastectomy as dissection methodology instead of electrocautery uses. It is well investigated the correlation between diathermal dissection and complication risk. Some scientific articles confirming that electrocautery dissection increases the chance of subdermal plexus thrombosis predisposing to skin flap failure (26).
In our previous experience in NSM, skin flaps less than 5 mm thick corresponded closely with development of major complications such as partial necrosis and implant failure. Flaps thicker than 5 mm improve prosthetic coverage, leading to better results. In contrast, flaps thicker than 10 mm are problematic, making lymphatic drainage difficult and culminating in cellulitis (2). So, in the present study, we have considered a 5–8 mm flap and NAC thickness advocating oncologic safety and blood supply.
Exclusion criteria were selected according with literature; patients with advanced disease that require neoadjuvant chemotherapies and/or radiation therapy were excluded in our patients series. Medical oncologist therapies are notable risk factors for adverse outcomes in healing, especially after DTI breast reconstruction, and place more stress on the breast skin viability after NSM (27,28). Smoking patients were also excluded from our series; smoking is an important risk factor for NAC necrosis, literature report a significant association between smokers and NAC/flap necrosis in subcutaneous mastectomies (29,30).
Women with BMI greater than 25 kg/m2 presents a higher risk of skin suffering as reported by Davies (31) and Platt also showed a high rate of major complication related to ischemia for patients with a BMI higher than 30 kg/m2 (32). Large and voluminous breast determines an increase of the length of the skin flap between the thoracic wall and the NAC reducing the blood supply of the edge of the breast (7). Therefore, the volume of the breast is hypothesized as a risk factor of necrosis of the NAC area. So we included in our series patients with BMI range between 18 to 30 kg/m2 and large size breasts were excluded for a safe NSM and DTI breast reconstruction.
Statistical analysis reported in literature showed a lower risk of flap failure in patient with age below 45 years (30); August report that complication rate for NSM and implant-based breast reconstruction in older women was less than in younger women (33). So the last exclusion criteria considered was age. We included patients younger than 65 years old.
DTI breast reconstruction nowadays is the best reconstruction option in conservative mastectomies. It eliminates the expansions and obviates the planned second stage breast reconstruction. So, physical and psychological impact of mastectomy is decreased accelerating a return to normal life with a restored body image and improved quality of life (34,35).
Acellular dermal matrices (ADMs) were adopted to solve a lot of surgical challenges. ADM were used in abdominal wall surgery (36), facial surgery (37), and neurosurgery (38). In 2001, Duncan reported the first use of ADM in breast surgery in both aesthetic and reconstructive cases (39). In 2006, Salzberg reported experience using ADM to achieve a single-stage implant reconstruction with full implant coverage (40). Since then, a lot of devices have been developed for DTI breast reconstruction. Implant based breast reconstruction with ADM/meshes present an implant failure rate up to 48% as reported in the literature. Causes of explantation are secondary to wound healing problems, persistent seroma formation, infection and large flap necrosis (2). Literature report optimal outcomes by considering strict patient selection (35).
In our series, in medium breasts for DTI breast reconstruction, we have used SurgiMend PRS (TEI, Biosciences, Inc.); so the implant was inserted into the newly created submuscular/ADM pocket for a total breast implant coverage. Regarding DTI after NSM, our properly surgical procedure and exclusion criteria, played an active role to decrease major complications rate and implant failure as demonstrated by our encouraging results.
The limitations of our study include the small subject numbers and its retrospective nature, which has the potential for bias. We retain that prospective studies provide greater significance and importance and a higher level of evidence; a longer follow-up is necessary for evaluating the pitfalls and limitations and defining finally the right and safe indications and execution of NSM and DTI breast reconstruction.
Conclusions
NSM and DTI breast reconstruction is a psychologically effective and safe prophylactic and therapeutic procedure (16). A careful preoperative and intraoperative surgical methodology, as proposed, is mandatory to reduce the complications rate. Oncoplastic approach by a single surgeon is encouraged to maximize the benefit and outcome of NSM and DTI breast reconstruction. Thanks to our previous reports (2,35,41) and the present study corroborated by LA-ICGA, we consider in our hands the procedure described safe and reproducible in terms of oncologic purposes and results. Our experience serves as an impetus to declare that the oncoplastic procedure must to be performed by a single surgeon from the planning to the execution of the procedure, both oncologic and reconstructive as a part of the breast cancer treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval was not required considering the well renowned Intraoperative LA-ICGA technique and the oncoplastic procedure described, the study was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). A proper written informed consent was obtained from all the patients.
References
- Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol 2014;21:100-6. [Crossref] [PubMed]
- De Vita R, Zoccali G, Buccheri EM, et al. Outcome evaluation after 2023 Nipple-Sparing Mastectomies: Our Experience. Plast Reconstr Surg 2017;139:335e-47e. [Crossref] [PubMed]
- Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg 2012;68:446-50. [Crossref] [PubMed]
- Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg 2011;212:686-93. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 Breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five-year experience at the European Institute of Oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [Crossref] [PubMed]
- Chirappapha P, Petit JY, Rietjens M, et al. Nipple sparing mastectomy: does breast morphological factor related to necrotic complications? Plast Reconstr Surg Glob Open 2014;2:e99. [Crossref] [PubMed]
- Diep GK, Hui JY, Marmor S, et al. Postmastectomy Reconstruction Outcomes After Intraoperative Evaluation with Indocyanine Green Angiography Versus Clinical Assessment. Ann Surg Oncol 2016;23:4080-5. [Crossref] [PubMed]
- Sood M, Glat P. Potential of the SPY intraoperative perfusion assessment system to reduce ischemic complications in immediate postmastectomy breast reconstruction. Ann Surg Innov Res 2013;7:9. [Crossref] [PubMed]
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Freeman BS. Complications of subcutaneous mastectomy with prosthetic replacement, immediate or delayed. South Med J 1967;60:1277-80. [Crossref] [PubMed]
- Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: Can we predict the factors predisposing to necrosis? Eur J Surg Oncol 2012;38:125-9. [Crossref] [PubMed]
- Petit JY, Veronesi U, Rey P, et al. Nipple-sparing mastectomy: Risk of nipple-areolar recurrences in a series of 579 cases. Breast Cancer Res Treat 2009;114:97-101. [Crossref] [PubMed]
- Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg 2011;19:129-33. [Crossref] [PubMed]
- Lohsiriwat V, Petit J. Nipple Sparing Mastectomy: from prophylactic to therapeutic standard. Gland Surg 2012;1:75-9. [PubMed]
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: A comprehensive review of the literature. Plast Reconstr Surg 2013;131:969-84. [Crossref] [PubMed]
- Johnson CH, vanHeerden JA, Martin JK, et al. Oncological aspects of immediate breast reconstruction following mastectomy for malignancy. Arch Surg 1989;124:819-23. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple-sparing mastectomy in association with intra operative radiotherapy (ELIOT): A new type of mastectomy for breast cancer treatment. Breast Cancer Res Treat 2006;96:47-51. [Crossref] [PubMed]
- Rusby JE, Brachtel EF, Taghian A, et al. George Peters Award. Microscopic anatomy within the nipple: Implications for nipple-sparing mastectomy. Am J Surg 2007;194:433-7. [Crossref] [PubMed]
- Irwin GW, Black A, Refsum SE, et al. Skin-reducing mastectomy and one-stage implant reconstruction with a myodermal flap: a safe and effective technique in risk-reducing and therapeutic mastectomy. J Plast Reconstr Aesthet Surg 2013;66:1188-94. [Crossref] [PubMed]
- Harless CA, Jacobson SR. Tailoring through Technology: A Retrospective Review of a Single Surgeon's Experience with Implant-Based Breast Reconstruction before and after Implementation of Laser-Assisted Indocyanine Green Angiography. Breast J 2016;22:274-81. [Crossref] [PubMed]
- De Lorenzi F, Yamaguchi S, Petit JY, et al. Evaluation of skin perfusion after nipple-sparing mastectomy by indocyanine green dye. Preliminary results. J Exp Clin Cancer Res 2005;24:347-54. [PubMed]
- Rinker B. A Comparison of Methods to Assess Mastectomy Flap Viability in Skin-Sparing Mastectomy and Immediate Reconstruction: A Prospective Cohort Study. Plast Reconstr Surg 2016;137:395-401. [Crossref] [PubMed]
- Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg 2010;26:487-92. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: Predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- Féron JG, Leduey A, Mallon P, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Ann Chir Plast Esthet 2014;59:333-43. [PubMed]
- Alperovich M, Choi M, Frey JD, et al. Nipple-sparing mastectomy in patients with prior breast irradiation: Are patients at higher risk for reconstructive complications? Plast Reconstr Surg 2014;134:202e-6e. [Crossref] [PubMed]
- Dolen UC, Schmidt AC, Um GT, et al. Impact of neoadjuvant and adjuvant chemotherapy on immediate tissue expander breast reconstruction. Ann Surg Oncol 2016;23:2357-66. [Crossref] [PubMed]
- Komorowski AL, Zanini V, Regolo L, et al. Necrotic complications after nipple- and areola-sparing mastectomy. World J Surg 2006;30:1410-3. [Crossref] [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [Crossref] [PubMed]
- Davies K, Allan L, Roblin P, et al. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast 2011;20:21-5. [Crossref] [PubMed]
- Platt AJ, Mohan D, Baguley P. The effect of body mass index and wound irrigation on outcome after bilateral breast reduction. Ann Plast Surg 2003;51:552-5. [Crossref] [PubMed]
- August DA, Wilkins E, Rea T. Breast reconstruction in older women. Surgery 1994;115:663-8. [PubMed]
- Govshievich A, Somogyi RB, Brown MH. Conservative mastectomies and immediate reconstruction with the use of ADMs. Gland Surg 2015;4:453-62. [PubMed]
- De Vita R, Buccheri EM, Pozzi M, et al. Direct to implant breast reconstruction by using SERI, preliminary report. J Exp Clin Cancer Res 2014;33:78. [Crossref] [PubMed]
- Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188-94. [Crossref] [PubMed]
- Shorr N, Perry JD, Goldberg RA, et al. The safety and applications of acellular human dermal allograft in ophthalmic plastic and reconstructive surgery: A preliminary report. Ophthal Plast Reconstr Surg 2000;16:223-30. [Crossref] [PubMed]
- Chaplin JM, Costantino PD, Wolpoe ME, et al. Use of acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 1999;45:320-7. [Crossref] [PubMed]
- Duncan DI. Correction of implant rippling using allograft dermis. Aesthet Surg J 2001;21:81-4. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- De Vita R, Pozzi M, Zoccali G, et al. Skin-reducing mastectomy and immediate breast reconstruction in patients with macromastia. J Exp Clin Cancer Res 2015;34:120. [Crossref] [PubMed]


