Sexuality, fertility and pregnancy following breast cancer treatment
Introduction
Women diagnosed and treated for breast cancer face a substantial risk that their sexual health will be negatively impacted. For women of reproductive age, the detrimental impacts of treatment on fertility and their ability to carry a pregnancy (and their future as mothers) are well described and are of paramount interest to this population. In this section, we will highlight issues related to these three issues for women following treatment of breast cancer. These issues will be discussed separately, emphasizing that each topic is unique in and of itself, as are the potential therapeutics and modes of prevention that are available.
Sexual health
Sexual health is an important aspect of quality of life and has been consistently rated as a significant area of concern, yet one which has gone relatively unaddressed. This was shown in the 2006 Livestrong survey that included over 2,000 cancer survivors who were predominantly female (1). Almost half of respondents reported concerns about sexual function making it the third most frequently noted physical concern, yet the majority (70%) did not seek out medical care or advice. Among those who noted detrimental changes to sexual health after cancer, significant emotional concerns were also reported, including depression, negative views of their own personal appearance, the experience of stigma, and difficulties in personal relationships. For women, sexual function can be construed as a multi-domain construct, as proposed by Rosemary Basson, consisting of intimacy, sexual stimuli, arousal, desire, and satisfaction (2). An additional aspect of sexual health has been proposed by Gass et al. comprised of breast specific sensuality (BSS) (3). At least one study showed that among a community sample of women, compared to other models of sexual function (e.g., Masters and Johnson and the Kaplan model), Basson’s model better correlated with sexual dysfunction scores on at least one validated tool (the Female Sexual Function Index, FSFI) (4).
Beyond global sexual dysfunction, another construct has emerged in the literature with particular relevance to women treated for breast cancer. Gass and colleagues have proposed a concept of BSS which she defined as the way a woman defines her breasts’ role during intimacy (3). Using a convenience sample of almost 300 women queried during an outpatient visit, almost all women felt their breast was a part of intimacy prior to surgery (83% of those who underwent lumpectomy, 95% of those choosing mastectomy, and 93% of those who chose mastectomy and reconstruction). Following surgery, there was a statistically significant decline in BSS regardless of surgery (74%, 47%, and 77%, respectively). Compared to those who chose mastectomy with reconstruction, higher FSFI scores among women who underwent a lumpectomy were significantly associated with more satisfaction in appearance and of BSS during breast caress.
Perhaps the strongest argument in favor of addressing sexual health issues is the potential to provide a positive intervention, and such interventions can be considered in two primary boxes: those to treat the genitourinary symptoms of menopause (GSM) and those to increase pleasure during sexual activities (Table 1). For the treatment of GSM, a non-hormonal option should be tried first. In our practice we often will utilize routine use of vulvovaginal moisturizers, such as polycarbophil or parabens-free preparations. Although not specifically aimed at improving sexual function, a randomized trial of women complaining of dyspareunia and vaginal dryness, compared to placebo, a greater proportion of patients had improvement in dyspareunia scores with the use of a moisturizer (60% vs. 41%, P=0.05) (5). For postmenopausal women, dehydroepiandrosterone (DHEA) may also provide benefit. This was shown in the NCCTG N10C1 (Alliance) trial that included 464 women with a history of breast or a gynecological cancer (6). Compared to plain moisturizer (PM) and to a lower dose (DHEA 3.5 mg daily), DHEA (6.5 mg daily) significantly improved sexual health based on FSFI scores. In a second publication, investigators also reported that DHEA increased serum testosterone levels in a dose-dependent manner compared to PM, and it increased estradiol levels when the highest dose was administered (DHEA 6.5 mg/d) though this was not seen among women taking an aromatase inhibitor (AI) (7). Such data are reassuring that DHEA may be an appropriate treatment for GSM with a secondary benefit to improving overall sexual health. Most recently, interest has been generated with the use of laser therapies using fractional carbon dioxide. However, there are only limited data to guide its use in this population and should be evaluated in the context of a randomized trial before its use can be endorsed.
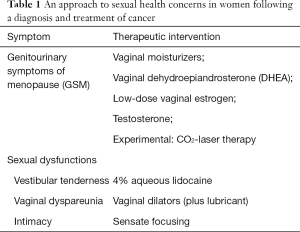
Full table
For women who persist with symptoms despite moisturizers, vaginal hormonal preparations can be effective without a significant risk for subsequent breast cancer events. LeRay et al. evaluated the safety of local estrogen therapies in a study that compared women who had relapsed from breast cancer (n=917) to those who remained without evidence of disease (n=8,885) (8). They reported that there was no significant difference in relative risk of a recurrence if estrogen therapy was used concomitantly (RR =0.78; 95% CI, 0.48–1.25) or sequentially (RR =0.97; 95% CI, 0.22–4.18) with endocrine treatment. Melisko et al. conducted a randomized trial of 12 weeks of intravaginal testosterone cream (IT) vs. an estradiol ring (ER) among women with early stage breast cancer taking an AI (9). Both preparations were associated with improvements in vaginal atrophy and sexual health endpoints (interest and overall dysfunction). Importantly, both treatments were noted to be safe; none of the patients using ER had persistent elevations in estradiol (E2) and only 4 of 34 (12%) of women exhibited this using IT. Finally, the use of estrogen therapy locally has been endorsed by the American College of Obstetricians and Gynecologists although they do suggest it be reserved for use in patients who do not benefit from non-hormonal interventions rather than as a first-line choice (10).
For women with dyspareunia, pelvic examination is critical as tenderness can be present at the initial point of penetration (the vestibule) and/or within the vaginal cavity. For women with breast cancer who experience pain specifically at the vestibule, 4% aqueous lidocaine can be effective (11). In a randomized trial that included 46 breast cancer survivors, use of lidocaine (applied topically to the vestibule for 3 minutes prior to planned penetrative activities) resulted in less dyspareunia and after open-label use, 90% reported a return to comfortable penetration. It should be noted, however, that lidocaine would not be adequate treatment for woman who complain of pain beyond the vestibule. For such women, we rely on vaginal dilators as a means of improving vaginal stretch, although we must acknowledge the lack of evidence to show if there is a benefit to treatment. Dilators come in graded size and width and women should be instructed to use the smallest most comfortable size at first, and only going up in size when they are comfortable. The use of good lubricant is critical for treatment, but even with this, discontinuation of use is quite common.
Lastly, it is important to recognize how breast cancer not only impacts the patient but her partner and as a result, couples often must grapple with adjusting to life together after treatment ends. Indeed, communication styles and perceptions of responsiveness have been found to mediate post-diagnostic intimacy (12). Intimacy exercises, including the use of sensate focusing, may help patients and their partners adjust to life after breast cancer, when it might be different, but does not have to be worse than before.
Addressing fertility
Breast cancer is the most commonly diagnosed malignancy in women of reproductive age with up to 15% occurring before the age of 40 years (13). While improvement in systemic therapies have contributed to improved 5-year survival rates (up to 91% in women newly diagnosed) (14), treatment for breast cancer places reproductive age women at a real risk of reduced fertility or infertility. Hence, it is important that interventions be provided early, preferably prior to the exposure to potentially gonadotoxic treatment.
Although not a perfect surrogate for fertility, amenorrhea has often been used to gage risk and the resumption of menses to gage reproductive health. In this vein, the risk of chemotherapy-related amenorrhea is largely related to age and type of cytotoxic treatment received (15). Chemotherapy can affect this reserve by directly inducing damage to the ovary and the magnitude of damage is inversely related to age with younger women (who have a greater ovarian reserve) at lower risk compared to older women (16). Some data suggest that one population of young women that may be at particularly high risk of infertility with systemic chemotherapy are those that harbor a BRCA gene mutation (17).
Among chemotherapeutics, alkylating agents cause the greatest potential harm to future fertility as they are non-cell-cycle dependent and can affect both actively dividing cells and non-active oocytes and pre-granulosa cells (18). Cyclophosphamide specifically is known to cause loss of ovarian follicle reserve, resulting in premature ovarian failure and infertility. Combination therapies such as cyclophosphamide, methotrexate, 5-fluorouracil (CMF) have been associated with a 21–71% chance of ovarian failure in those under 40 and upwards of 40–100% in those older than 41 (19). Other combinations with comparable cyclophosphamide doses such as cyclophosphamide-epirubicin-5-fluorouracil (CEF), cyclophosphamide-doxorubicin-5-fluorocuracil (CAF/FAC) show greater rates of ovarian toxicity at 1 year (40% in those under 40 vs. 90–100% in older than 40 years) (20).
Fortunately, modern regimens, such as doxorubicin-cyclophosphamide (AC), which are in more common use today has resulted in lower rates of gonadotoxicity (<20% under 40 years vs. 30–70% those older than 40) (21). A 2006 study evaluating taxane-anthracycline regimens reported a lower incidence of chemotherapy-induced amenorrhea compared to historical regimens where a larger amount of total cyclophosphamide was administered (22). As of now, the risk of targeted therapy is as yet not well defined. However, interest in understanding the role biologics play in fertility is growing. As an example, one study looking at combination chemotherapy-trastuzumab and the association to amenorrhea (23). Among their results, they did not find an increased risk of amenorrhea with the regimen of doxorubicin, cyclophosphamide followed by paclitaxel (AC + T) with or without trastuzumab (OR =0.6; 95% CI, 0.22–1.61).
Since systemic therapy threatens future fertility and many women have delayed child bearing for various reasons, fertility preservation is a desirable option and should be discussed with all appropriate patients prior to the start of therapy. The American Society of Clinical Oncology (ASCO) have published guidelines for preservation recommends routine fertility evaluation and counselling be offered as part of education and informed consent before cancer therapy with all reproductive-aged patients treated, including the option of a prompt referral to a reproductive specialist (24). For women with newly diagnosed breast cancer, established preservation therapies endorsed by ASCO and ASRM include embryo cryopreservation and oocyte cryopreservation. However other methods such as temporary ovarian suppression, ovarian tissue cryopreservation and transplantation should also be discussed, if not by oncologists, then by reproductive endocrinologists.
Embryo cryopreservation
Embryo cryopreservation entails the administration of exogenous hormones for ovarian stimulation (OS), collection of oocytes which are then fertilized using in vitro techniques, and the subsequent freezing of embryos (25). Despite a relatively lower success rate (compared to the use of fresh embryos at the time of implantation), success rates have been found to be similar in those with breast cancer compared to age-matched controls (26).
For women undergoing OS, whether it poses a risk to prognosis has been a chief concern for patients and for their physicians. In one literature review, Rodgers et al. reported that the available data supported the co-administration of an aromatase inhibitor on day 2 during hyperstimulation was not associated with an increased incidence of breast cancer recurrence compared to women who did not undergo OS (5% in both arms with a medium follow-up of 5 and 6.9 years, respectively) (27). In addition, co-administration resulted in suppression of estradiol levels without a reduction in the yield of oocytes.
Beyond this, a concern has also been raised about the potential to delay the start of systemic therapy by 2 to 6 weeks (28). However, at least one retrospective study suggested that for women scheduled to begin neoadjuvant chemotherapy, there was no significant difference in the time from diagnosis to initiation of treatment in women who underwent OS compared to women who did not (29).
For women who have a known genetic mutation that conferred personal risk of breast cancer, the option of pre-implanting genetic diagnosis (PGD) is available. This testing allows evaluation of embryos for mutations in the BRCA genes, which may aide in the selection of healthy embryos prior to eventual implantation (17).
Oocyte cryopreservation
Oocyte cryopreservation is an alternative method that is available at a number of centers, is favorable in women who are not partnered and is an option for women who have ethical concerns about embryo cryopreservation. The process consists of hormonal follicular maturation with subsequent harvesting and freezing. At present, however, it appears to be less effective than embryo cryopreservation in achieving a clinical pregnancy (18,30).
Oocytes can be sensitive to the chilling and thawing process, as crystallization can occur leading to damage of the mitotic spindle. Additionally, the zona pellucida can harden and inhibit fertility. New techniques such as rapid cooling or vitrification with a cryoprotectant solution have improved survival of oocytes (30). There has not been a notable difference between frozen and fresh oocyte cryopreservation (31).
GNRH agonists
The administration of gonadotropin releasing hormone (GNRH) agonists has been evaluated as a means of preserving ovarian function among women undergoing chemotherapy. Support for this intervention comes from an individual patient meta-analysis presented at the 2017 San Antonio Breast Cancer Symposium that included 5 randomized trials (n=873 patients) (32). Compared to chemotherapy alone, the use of a GnRHa significantly reduced the rate of premature ovarian insufficiency (POI, 14% vs. 30.9%; adjusted OR =0.38; 95% CI, 0.26–0.57). In addition, patients achieving at least one post-treatment pregnancies were higher in the group who received a GnRH (37 vs. 20, P=0.03), but there was no significant differences reported in survival outcomes.
Other
Other methods of fertility preservation are currently felt to be experimental, however have not been adequately evaluated in those with breast cancer. Such methods include: ovarian tissue cryopreservation and transplantation. While successful pregnancies have been witnessed, around 19 live births to date, the theoretical concern remains with reimplanting ovarian tissue with potential of reintroducing cancer cells (24).
Pregnancy
Most clinicians advise women completing initial diagnosis and treatment for breast cancer to wait at least 2 years before becoming pregnant based on this time period being associated with the highest risk of recurrence. However, there is no published data to support this recommendation. The only established guidelines are from the Society of Obstetricians and Gynaecologists of Canada from 2002, recommending waiting at least 3 years before attempting pregnancy, 5 years if there is nodal involvement (33). As an unfortunate result, this lack of data surrounding the timing pregnancy after BC have contributed to an induced abortion rate around 30% (34).
Multiple studies have shown pregnancy after BC to have no increased risk of recurrence and perhaps even a protective effect. A large meta-analysis of four large registry-based series that included 1,244 pregnancies after BC compared to over 18,000 controls showed that waiting 2 years or more had no impact on disease free survival compared to matched controls (35). Interesting, BC patients that became pregnant within 2 years of their BC diagnosis had a better disease free survival, suggesting that becoming pregnant after treatment for breast cancer may even have a protective effect with a decreased (35) risk of death of 41%. This effect was not significantly modified by age at diagnosis, tumor size, nodal status, or reproductive history before a diagnosis of BC. Results may be biased by what is termed a “healthy mother effect”, proposing that women with a diagnosis of breast cancer who subsequently conceive are a self-selecting group of women with a better prognosis (36). A meta-analysis attempting to overcome the healthy mother bias showed that overall survival was statistically higher among patients who became pregnant at least 10 months after a BC diagnosis than amongst those who did not (37). A third meta-analysis looking at the safety of pregnancy after surgical treatment for BC noted no increase in recurrence rates and a probable improvement in overall survival (38). A separate study specifically looked at the risk for women with an endocrine-sensitive breast cancer and reported that although endocrine sensitive tumors did confer a protective effect within the first 5 years after pregnancy it did not appear to have a detrimental effect on recurrence (34).
For women with a HER2-positive breast cancer, we suggest deferring pregnancy until all treatment has completed, especially given the improvement in disease-free and overall survival with HER2 based therapies. In addition, timing should take in to account the individual risk of relapse, with some experts suggesting that for women at high risk, deferring pregnancy for up to 5 years should be considered. Waiting as long as 5 years may be advised in women with a high risk of relapse (33,39). However, for those at low risk, waiting as little as 6 months may be safe. Although data are limited, one study in Australia included the outcomes of 62 women who conceived pregnancy less than 2 years after a breast cancer diagnosis (40). While waiting at least 2 years before conception was significantly associated with a survival advantage, women who waited at least 6 months also experienced a protective effect (HR =0.48; 95% CI, 0.27–0.83), although it was not a statistically significant result.
In summary, there are no established guidelines regarding the timing of conception after diagnosis and treatment of breast cancer. Studies are suggestive of a protective effect of pregnancy and improved survival in women treated with BC compared to matched controls. Newer therapies that came out after many of the women in these studies were treated may improve prognosis further. Waiting until the completion of adjuvant therapies may be advised, especially for women at higher risk of recurrence. However, decisions must be weighed against the risk of advanced maternal age and a lower success of becoming pregnant after treatment for breast cancer. A global prospective clinical trial by The Breast International Group (BIG) and North American Breast Cancer Group (NABCG) Endocrine Working Group (the POSITIVE trial) is looking at the feasibility and impact of a temporary treatment interruption of endocrine therapy to allow conception (41).
Conclusions
With improvements in screening, detection, and treatment, most women diagnosed with breast cancer can have a good prognosis. Hence, the attention to quality of life is as important as the administration of effective systemic therapies. Recognizing that treatment can have life-altering impacts for women and their partners is essential, as is the provision of education and options (for treatment and for specialty referral).
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Dizon is a Section Editor for UpToDate and serves on the Board of Directors for the Patty Brisben Foundation.
References
- Challenges Reported by Post-Treatment Cancer Survivors in the LIVESTRONG Surveys | What We Do. Available online: http://www.livestrong.org/what-we-do/our-approach/reports-findings/survivor-survey-report/. Accessed 12/5/2017.
- Basson R, Wierman ME, van Lankveld J, et al. Summary of the recommendations on sexual dysfunctions in women. J Sex Med 2010;7:314-26. [Crossref] [PubMed]
- Gass JS, Onstad M, Pesek S, et al. Breast-Specific Sensuality and Sexual Function in Cancer Survivorship: Does Surgical Modality Matter? Ann Surg Oncol 2017;24:3133-40. [Crossref] [PubMed]
- Sand M, Fisher WA. Women’s endorsement of models of female sexual response: the nurses’ sexuality study. J Sex Med 2007;4:708-19. [Crossref] [PubMed]
- Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol 1997;15:969-73. [Crossref] [PubMed]
- Barton DL, Sloan JA, Shuster LT, et al. Evaluating the efficacy of vaginal dehydroepiandosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance). Support Care Cancer 2018;26:643-50. [Crossref] [PubMed]
- Barton DL, Shuster LT, Dockter T, et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer 2018;26:1335-43. [Crossref] [PubMed]
- Le Ray I, Dell’Aniello S, Bonnetain F, et al. Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat 2012;135:603-9. [Crossref] [PubMed]
- Melisko ME, Goldman ME, Hwang J, et al. Vaginal Testosterone Cream vs Estradiol Vaginal Ring for Vaginal Dryness or Decreased Libido in Women Receiving Aromatase Inhibitors for Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA Oncol 2017;3:313-9. [Crossref] [PubMed]
- American College of Obstetricians and Gynecologists. The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Committee Opinion Number 659. Available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/The-Use-of-Vaginal-Estrogen-in-Women-With-a-History-of-Estrogen-Dependent-Breast-Cancer. Accessed 12/17/2017.
- Goetsch MF, Lim JY, Caughey AB. A Practical Solution for Dyspareunia in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol 2015;33:3394-400. [Crossref] [PubMed]
- Rowland JH, Meyerowitz BE, Crespi CM, et al. Addressing intimacy and partner communication after breast cancer: a randomized controlled group intervention. Breast Cancer Res Treat 2009;118:99-111. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Lee MC, Gray J, Han HS, et al. Fertility and reproductive considerations in premenopausal patients with breast cancer. Cancer Control 2010;17:162-72. [Crossref] [PubMed]
- Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 1999;17:2365-70. [Crossref] [PubMed]
- Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev 2017;59:61-70. [Crossref] [PubMed]
- Partridge AH, Ruddy KJ. Fertility and adjuvant treatment in young women with breast cancer. Breast 2007;16:S175-81. [Crossref] [PubMed]
- Camp-Sorrell D. Cancer and its treatment effect on young breast cancer survivors. Semin Oncol Nurs 2009;25:251-8. [Crossref] [PubMed]
- Parulekar WR, Day AG, Ottaway JA, et al. Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: analysis of a National Cancer Institute of Canada Clinical Trials Group Study--NCIC CTG MA.5. J Clin Oncol 2005;23:6002-8. [Crossref] [PubMed]
- Christinat A, Pagani O. Fertility after breast cancer. Maturitas 2012;73:191-6. [Crossref] [PubMed]
- Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol 2006;24:1045-51. [Crossref] [PubMed]
- Abusief ME, Missmer SA, Ginsburg ES, et al. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer 2010;116:791-8. [Crossref] [PubMed]
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500-10. [Crossref] [PubMed]
- Shapira M, Raanani H, Meirow D. IVF for fertility preservation in breast cancer patients--efficacy and safety issues. J Assist Reprod Genet 2015;32:1171-8. [Crossref] [PubMed]
- Cardozo ER, Thomson AP, Karmon AE, et al. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet 2015;32:587-96. [Crossref] [PubMed]
- Rodgers RJ, Reid GD, Koch J, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod 2017;32:1033-45. [Crossref] [PubMed]
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917-31. [Crossref] [PubMed]
- Chien AJ, Chambers J, Mcauley F, et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II-III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat 2017;165:151-9. [Crossref] [PubMed]
- Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet Gynecol Surv 2007;62:58-72. [Crossref] [PubMed]
- O’Donoghue C, Quinn GP, Lee MC. Fertility Preservation in Breast Cancer. South Med J 2017;110:621-6. [Crossref] [PubMed]
- Lambertini M, Moore HC, Leonard RCF, et al. Pooled analysis of five randomized trials investigating temporary ovarian suppression with gonadotropin-releasing hormone analogs during chemotherapy as a strategy to preserve ovarian function and fertility in premenopausal early breast cancer patients. 2017 San Antonio Breast Cancer Symposium. Available online: www.abstracts2view.com/sabcs/view.php?nu=SABCS17L_1141&terms=. Accessed Dec 8, 2017.
- Helewa M, Lévesque P, Provencher D, et al. Breast cancer, pregnancy, and breastfeeding. J Obstet Gynaecol Can 2002;24:164-80. [Crossref] [PubMed]
- Azim HA, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol 2013;31:73-9. [Crossref] [PubMed]
- Azim HA, Santoro L, Pavlidis N, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer 2011;47:74-83. [Crossref] [PubMed]
- Sankila R, Heinävaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: “healthy mother effect. Am J Obstet Gynecol 1994;170:818-23. [Crossref] [PubMed]
- Valachis A, Tsali L, Pesce LL, et al. Safety of pregnancy after primary breast carcinoma in young women: a meta-analysis to overcome bias of healthy mother effect studies. Obstet Gynecol Surv 2010;65:786-93. [Crossref] [PubMed]
- Luo M, Zeng J, Li F, et al. Safety of pregnancy after surgical treatment for breast cancer: a meta-analysis. Int J Gynecol Cancer 2014;24:1366-72. [Crossref] [PubMed]
- Largillier R, Savignoni A, Gligorov J, et al. Prognostic role of pregnancy occurring before or after treatment of early breast cancer patients aged <35 years: a GET(N)A Working Group analysis. Cancer 2009;115:5155-65. [Crossref] [PubMed]
- Ives A, Saunders C, Bulsara M, et al. Pregnancy after breast cancer: population based study. BMJ 2007;334:194. [Crossref] [PubMed]
- Pagani O, Ruggeri M, Manunta S, et al. Pregnancy after breast cancer: Are young patients willing to participate in clinical studies? Breast 2015;24:201-7. [Crossref] [PubMed]


