Overview of indications for nipple sparing mastectomy
Introduction
The trend in breast surgery over the past century has been toward a more minimally invasive approach with better cosmetic outcomes. The introduction of more targeted systemic therapies, better screening modalities with earlier diagnosis and dramatically improved reconstructive techniques has allowed these advancements. The recent introduction of nipple sparing mastectomy (NSM) has dramatically improved the cosmetic outcomes and quality of life (QoL) for patients undergoing mastectomy. This technique involves preservation of both the skin envelope including the nipple areolar complex commonly through a barely visible inframammary skin incision followed by immediate breast reconstruction. First pioneered by Freeman in 1962, the procedure was fraught with complications, poor cosmetic outcomes and questions about its oncologic safety (1). It was therefore not widely used by surgeons. The procedure regained popularity after Hartmann et al. in 1999 published their Mayo Clinic experience with prophylactic mastectomy in high risk women. The majority of women in this study had undergone NSM. Only a small minority about 1% (7/693) in this group developed a subsequent breast cancer and there was no difference in risk reduction whether the nipple was excised or retained (2,3).
More recently, in the last 20 years, several retrospective studies on NSM have proven the oncologic safety of this procedure. Therefore, the inclusion criteria for women who are candidates for NSM have widely expanded. The initial guidelines excluded patients with previous radiation, ptosis, high BMI and macromastia. These contraindications are now being challenged. Some newer approaches to these complex cases and recent QoL studies have shown positive outcomes in these challenging patients. NSM is now feasible in a larger subset of patients. Currently, only women with inflammatory breast cancer and nipple involvement are absolute contraindications for performing a NSM.
Indications
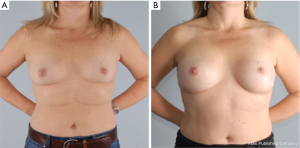
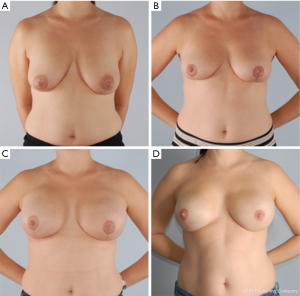
The goal of NSM is to obtain negative margins while achieving an excellent cosmetic outcome. At our institution we consider patients ideal for this procedure if they have no or minimal ptosis (grade 0 or 1), A or B cup breast size, a BMI <30 kg/m2 and not an active smoker. The patient in Figure 1 demonstrates an ideal NSM candidate with small breasts and no ptosis. Patient proceeded with bilateral NSM, followed by direct to prepectoral implant at the same operation. Tissue expanders were unnecessary due to intraoperative verification of healthy skin flaps using the Spy Machine.
Non ideal patients that are at high risk of having a postoperative complication or inferior cosmetic outcomes are those patients with grade 2 or 3 ptosis, macromastia (C cup breast size or larger), obesity (BMI >30 kg/m2), pre or post mastectomy radiation (pMRT/PMRT) are actively smoking at time of surgery.
Risk reduction in high risk patients and genetic carriers
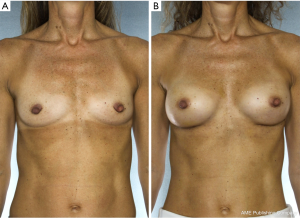
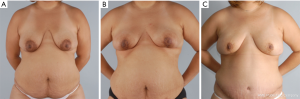
NSM for prophylactic purposes has been well accepted for years. However, NSM for the treatment of high risk genetic mutation carriers has been controversial due to the paucity of data with small numbers of patients in the existing studies and uncertain residual risk with nipple retention. Because the lifetime risk of breast cancer in BRCA1/2 carriers ranges from 60–80% with a 3–4% risk/year of contralateral breast cancer once the first event occurs, many women choose to undergo bilateral prophylactic mastectomy (4). While some genetic carriers choose close observation with clinical breast examinations, annual mammogram and magnetic resonance imaging (MRI) screening, preventative mastectomy patients reduce their overall risk of developing a future breast cancer by more than 90%. Figure 2 demonstrates a patient carrying a BRCA gene who underwent bilateral prophylactic mastectomies.
A recent retrospective review by Yao et al. is one of the largest studies to date on genetic carriers undergoing NSM. This study examined outcomes of 397 NSMs in 201 BRCA1/2 carriers from two different institutions between 2007–2014 who underwent NSM for both therapeutic and risk reduction purposes (4). Patients were candidates for NSM if they had no nipple-areolar complex (NAC) involvement, a negative axilla on clinical examination and negative preoperative imaging such as MRI, mammogram or ultrasound. The study also included patients with any breast size if the treating surgeons felt a favorable cosmetic result was feasible. One of the institutions expanded the eligibility criteria to include patients with clinically positive axillary nodes. One hundred and fifty patients (298 breasts) underwent NSM for risk reduction and 51 patients for cancer (99 breasts). Overall complication rates were low and comparable to non-carrier populations (4). Flap necrosis occurred in 10 (2.5%) and NAC loss occurred in 7 (1.8%) breasts, three due to cancer involvement (5.8%) and four from necrosis. Incidental cancer in prophylactic patients was found in only 4 of 120 patients (2.7%); none occurred at the nipple. Two of 51 (3.9%) patients had incidental cancers in the cancer treatment group; both were ductal carcinoma in situ in the contralateral breast. At a mean follow up of 32 months, there have been four new cancer events found in three cancer patients and one prophylactic patient 6 years later, but none at the nipple. In addition, only three BRCA1/2 cancer patients had tumor involvement of the NAC on frozen or permanent section, a rate similar to rates of NAC involvement in patients with sporadic cancers (4).
Incidental cancers reported for BRCA1/2 carriers in other series ranged from 0% (4,5) to 3% (4,6-8). For non-BRCA1/2 carriers the rates were similar and ranged from 0.1% (2,4) to 5.6% (4,9). Several outcome studies have proven the oncologic safety of NSM in high risk genetic patients both in the prophylactic and therapeutic setting. Although most studies examining BRCA positive patients undergoing therapeutic NSM contain small sample sizes, the outcomes are favorable. Memorial Sloan Kettering Cancer Center showed no recurrences in 14 patients at 10 months follow up (4,9). Peled et al. from the University of California San Francisco reported no local-regional recurrences at 4 years follow-up among 53 BRCA1/2 carriers who underwent NSM, 27 of whom had breast cancer, and no evidence of disease was found in the NAC at the time of surgery (4,10).
High risk patients who choose to undergo preventative NSM have had favorable outcomes in several studies. The prose study examined 2,482 BRCA1/2 carriers undergoing bilateral prophylactic mastectomy. Only one of these patients went on to develop cancer at 3 years follow up (4,11). In the very early part of that series, the surgical technique was subcutaneous mastectomy (3,4,8). At 6.4 years, 105 patients were still followed, of which 30% (29 breasts) underwent subcutaneous mastectomy. Two of 105 (1.9%) developed breast cancer, both of whom had undergone subcutaneous mastectomies, with one patient developing axillary metastasis and the other patients developing breast cancer in residual unspecified breast tissue (4,8). There was no statistically significant difference in the occurrence of cancer between the NSM and non-NSM groups (12).
There have been several retrospective studies of women with a strong family history of breast cancer that underwent prophylactic mastectomy. The hallmark study from Mayo Clinic published in 1999 examined 639 high risks women who underwent prophylactic NSM, of which approximately 90% had subcutaneous mastectomy. Only seven of these patients developed breast cancer after 14 years’ median follow-up (2,4). McDonnell reported on 745 women from the Mayo Clinic between 1960–1993 with a first breast cancer and a strong family history who underwent unilateral prophylactic mastectomy of the opposite breast. 41% of mastectomies were NSM, and of those women only 4 developed cancers, none of which developed near the nipple. Out of the remaining 59% of non-NSM patients, only 4 developed cancers that were also not in proximity to the nipple (12,13).
In 2006, Sacchini et al. conducted a large multi-institutional international study including patients from New York, Sao Paulo, Brazil, Milan and Padua, Italy. Of the 55 patients who underwent prophylactic NSM, there were no new cancers in the nipple with a mean follow up of 24 months. Two cancers did develop after prophylactic NSM: one in the axillary tail at 24 months, and one in the upper outer quadrant at 62 months (12,14). To date, several retrospective studies for women at high risk of breast cancer who undergo preventative mastectomy have consistently shown that NSM is an oncologically safe procedure in this setting.
NSM for breast cancer patients
Previous algorithms for selection of oncologically safe surgical candidates at our own institution, published in 2011, have already dramatically changed and widened. Previous criteria included tumor size <3 cm, tumor distance >2 cm from the nipple, clinically negative axillary nodes, no skin involvement or inflammatory cancer/Paget’s disease and possible preoperative MRI to exclude nipple involvement (12). This criteria was based on previous studies examining NSM and cancer recurrence rates, including Laronga [1999], Gerber and Krause [2003], Crowe [2004], Sacchini [2006], some of which excluded patients with previous neoadjuvant chemotherapy (12). Cancer recurrence was found in 6 (5.4%) of 112 NSM patients in the study by Gerber (12,15). Jensen et al. stated that based on the NSABP B-06 study comparing mastectomy, lumpectomy plus radiation, and lumpectomy alone, disease-free, distant disease-free and overall survival was the same regardless of whether the nipple was removed and therefore this could be extrapolated to NSM (12).
Early data examining nipple involvement from the 1970’s and 1980’s ranged from 0–58%. The methodology for tissue examination and criteria for involvement was unfortunately not uniform. Historically, the tumors were diagnosed later and more advanced (12,14). A study in 2006 by Brachtel et al. sought to identify frequency and patterns of occult nipple involvement in a large cohort of 316 consecutive mastectomy specimens (232 therapeutic, 84 prophylactic) with grossly unremarkable nipples (16). Nipples were excised with adjacent areolar and retroareolar tissue and evaluated by multiple coronal sections through the entire nipple and retroareolar tissue to a depth of at least 0.5 cm below the skin level. A retroareolar margin was defined as the tissue block below the areolar skin level. 71% of nipples (164) from therapeutic mastectomies showed no pathologic abnormality, 21% (49) had DCIS (majority), invasive carcinoma (IC), or lymphovascular invasion (LVI), and 8% LCIS. HER2 amplification, tumor size >2 cm, and tumor-nipple distance ≤4 cm were associated with nipple involvement by multivariate analysis (P=0.0047, 0.0126 and 0.0176); histologic grade of both DCIS (P=0.002) and IC (P=0.03), LVI (P=0.03) and lymph node involvement (P=0.02) by univariate analysis. None of the 84 prophylactic mastectomies showed nipple involvement by IC or DCIS. This analysis helped determine patient eligibility for NSM, and guide future NSM nipple margin studies.
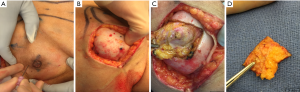
In a recent study by Coopey et al., the patients with bloody nipple discharge, locally advanced breast cancer with skin involvement, inflammatory cancer and women with imaging evidence of NAC involvement were excluded from NSM (17). Similar exclusion criteria are followed at our institution. MRI is routinely used preoperatively in NSM candidates to help assess distance of tumor to skin as well as nipple margin. If there is a clear dissection plane visualized on MRI preoperatively, the patient is considered a candidate for NSM. All additional nipple margins taken intraoperatively are sent for permanent sectioning. Patients are counseled preoperatively that if the nipple margin is positive, the current standard of care is removal of the nipple areolar complex to achieve negative margins and local control. At our institution, there is a subset of patients who have refused removal of a positive nipple areolar complex and have opted to undergo a shave biopsy from the posterior aspect of the nipple. If that additional shave biopsy shows additional disease, then the patient will undergo final excision of the nipple areolar complex. Our preliminary retrospective results show that of 40 patients with positive nipple margins from 2003–2017, 16 patients (40%) underwent shave biopsy of subareolar tissue. Of the shave biopsies, final pathology revealed one positive margin (DCIS within 1 mm of margin). No patients developed NAC necrosis following shave biopsy necessitating subsequent NAC removal. There were no recurrences with a mean follow up time of 2.4 years. For patients who have undergone placement of a tissue expander with a subsequent positive nipple margin, NAC removal or shave margin can be safely performed at the time of their expander exchange to final implant avoiding an additional operation. After close collaboration with the reconstructive surgeon, a unique technique demonstrated in Figure 3 is carried out for those patients who wish to undergo an additional shave margin. A small dot of methylene blue is injected intraoperatively using a 27 gauge needle at the borders of the NAC in 4 locations in the skin, which then delineates the borders on the posterior aspect of the NAC from the inside. This allows for a clearly demarcated area where shave margins should be excised from the back of the NAC. The implant is removed intraoperatively allowing access and replaced at the conclusion of the procedure.
In Coopey’s study, from June 2007 through December 2012, NSM was performed on 645 breasts in 370 patients. Patients operated on in the last two years [2011–2012] of the study period were examined separately to determine trends towards improved outcomes as experience was gained (17). Patients were not excluded based on tumor size or tumor to nipple distance. Nipple loss due to positive subareolar/nipple margins was significantly less in the 2011–2012 group (6.5% vs. 2.7%, P=0.027), even though a higher percentage of patients undergoing NSM in the 2011–2012 group had a cancer diagnosis, showing technical improvement (17). Two point seven percent nipple positivity is favorable compared to other reported rates of positive subareolar margins, which have historically ranged from 3% to 21% (9,10,17-20). At a mean follow-up of 22.1 months, local recurrence occurred in 4 of 156 (2.6%) breasts operated on for cancer through 2011, which is even lower than the older studies previously mentioned with strict selection criteria (17). Reported 5-year local recurrence rates range from 0% to 5% (17). The highest recurrence rate to date is 24% in a study by Benediktsson et al., however follow up was as long as 13 years (4,21). In Coopey’s study, there have been no local recurrences in patients operated on in 2012, no recurrences in the NAC and no cancer in prophylactic patients (17). In an update on Coopey’s study by Tang et al., including additional patients from 2012–2014, there were no recurrences in the nipple areolar complex with a median follow up of 36 months (22). In addition, their practice evolved to remove only the nipple and retain the areola for positive margins: from 2007–2011, 7/17 (41%) underwent nipple only excision compared to 14/22 (64%) in 2012–2014 (22). Other studies have shown that most recurrences do not occur in the nipple, as most recur as distant metastatic disease, or regionally in the axilla (9,19,23).
It is has been our practice to submit a separately submitted nipple margin described by Spear from the posterior aspect of the nipple (12). We currently do not routinely send the additional nipple margin for frozen, as this small specimen may be compromised during the freezing process and permanent evaluation is more sensitive. In a study by Alperovich et al. at NYU, 307 of 480 breasts were sent for frozen section. Biopsies were positive on permanent section in 3.9% (12/307); five were falsely negative on frozen, and seven were concordant (24). The seven concordant patients underwent excision of the nipple intraoperatively (24). Most surgeons routinely send the additional intraoperative nipple margin for permanent section to avoid false positives and inadvertently remove a normal nipple.
In the Cornell experience, they examined 325 NSMs from 2007–2011. Frozen section was performed on 188/325 NSMs (58%) and found to be 64% sensitive and 99% specific (23). 14% of nipple margins (29/208) from therapeutic NSM and no nipple margins (0/117) from prophylactic NSMs showed malignancy. Central tumor location and N2/N3 lymph node status were significantly associated with nipple margin positivity. Nine in 29 patients with positive nipple margins chose to retain their nipple and only one invasive cancer recurred in the saved nipple 36 months after therapeutic NSM. Forty percent (8/20) of the nipples that had been resected after positive margin contained residual cancer, while 60% did not (12/20) (23).
In Tang’s experience at Massachusetts General, 43 of 642 (6.7%) therapeutic and 3 of 684 (0.4%) prophylactic NSMs had positive nipple margins; 39 of 46 patients underwent nipple or nac excision and 11/39 excised nipples containing residual malignancy (28%), while 28/39 (72%) did not (22). Of the few studies pertaining to positive nipple margins after NSM for both therapeutic and prophylactic purposes, most centers have had similar experiences. At John Wayne Cancer Institute, 22/149 nipples (14%) were excised due to positive nipple margins (19). Of the three recurrences at a mean follow up of 60.2 months, all had biopsy proven subareolar disease and had undergone nipple removal at original mastectomy after frozen section. As stated previously, NYU contained positive nipple margins in 21/480 breasts (4.3%), with only 30% or 6 of 20 resected specimens containing residual pathology (24). At Memorial Sloan Kettering Cancer Center, 11 of 341 (3.1%) nipples contained positive margins, warranting further excision. At both NYU and Massachusetts General Hospital, the residual pathology in the nipple at re-excision was primarily DCIS (22,24).
Understanding the anatomy of the nipple also can also lead to successful NSM cosmetic outcomes. A study by Rusby et al. examined the relationship of duct microvasculature in relation to position of duct bundles. Forty-eight specimens were selected to examine nipple and duct bundle cross sectional areas, while the number of vessels were counted within the duct bundle and peripheral rim in 7 non-irradiated and 5 irradiated nipples (25). It was found that mean nipple diameter was 11.1 mm and duct bundle diameter 5.2 mm, and a 2 and 3 mm peripheral rim of nipple tissue would result in complete duct excision in 96% and 87% of sections, respectively. It was also shown that 29% of vessels are located in the duct bundle, in which a 2 mm rim contains 50% of vessels, while a 3 mm rim contains 66%. Similar proportions were seen in irradiated nipples. This study helped provide a strategy for performing NSM to balance preservation of blood supply while excising a large percentage of duct bundles.
Another study by Rusby showed that the majority of ducts form a central bundle that occupies 21–67% of the cross-sectional area of the papilla (26).The central bundle narrows to form a “waist” before the ducts disperse and widen into the breast parenchyma. Understanding of this configuration would allow surgical excision of the central duct bundle in cases in which it is deemed advisable to remove all ductal tissue, such as in the case of DCIS. The median number of duct orifices out of 129 nipples in the study was 23, and one nipple tip demonstrated 29 ducts arising from 15 orifices. 17% of nipples contained lobular tissue, suggesting that even in prophylactic mastectomies, it is important to excise the duct core. Beneath the skin, most ducts were very narrow, gradually becoming larger deeper within the nipple.
NSM and radiation
Previous radiation to the breast had been considered a contraindication to NSM due to fear of nipple ischemia from an already compromised bloody supply and the chronic sequelae of radiation such as skin fibrosis leading to poor cosmesis. This ideology was refuted by Reish et al., comparing outcomes of irradiated patients with non-irradiated patients undergoing NSM with immediate breast reconstruction (27). Success with skin-sparing mastectomy followed by radiation is limited due to poor healing of nipple reconstruction incisions leading to exposure of the implants, as well as flattening and shrinkage of the nipple projection. More patients who undergo NSM are candidates for single-stage reconstruction with implants because of the extra skin preservation to the breast and use of acellular dermal matrix. Few studies have addressed the effects of pre- or post-mastectomy radiation for patients undergoing NSM with immediate reconstruction in which the nipple is fully preserved.
In Reish’s review, 605 immediate breast reconstructions were performed following NSM (27). Of the reconstructions, 88 were treated with radiation therapy and 517 had no radiation therapy. Preoperative radiation therapy was administered in 43 and 45 received postmastectomy radiation therapy to the site of reconstruction, and mean follow up was 686 days. If the skin envelope was healthy at conclusion of NSM, direct to implant reconstruction was performed. If it was not healthy, a tissue expander was placed. Patients with visible radiation-induced changes of the skin and/or hard fibrous breasts were not considered candidates for immediate or delayed alloplastic reconstruction. If the patient desired autologous reconstruction and required radiation, a tissue expander was placed at the time of NSM, and free-flap was performed post-radiation.
The total nipple retention rate in patients with radiation therapy and nipple-sparing mastectomy was 90% (79 of 88), which was the majority of patients. The reconstruction failure rate defined as explantations for immediate complications or capsular contracture was 8%. There was a trend toward higher complications such as infections in patients with radiation, and there was a significantly higher rate of implant loss. Skin flap necrosis and complications requiring explantation occurred in both groups. Implant volume tended to be smaller in those patients who had undergone pMRT. In the author’s experience, there were fewer complications with direct to implant reconstruction over tissue expanders, since a surgical procedure was avoided. In addition, nipple position was well maintained post radiation (27).
A similar study by Tang et al. helped determine which radiated patients were at greater risk of complications. It was shown that pMRT, PMRT, age >55 years, breast volume ≥800 cm3, smoking and periareolar incisions were independent risk factors for complications requiring surgical revision (28). In irradiated breasts, complication rates were 13.4% without further risk factors and 17.5%, 50% and 66.7% when 1, 2 and ≥3 additional independent risk factors were present, respectively (P<0.001).
NSM with pMRT or PMRT therapy is an area of research that needs larger sample sizes and longer follows up, as well as patient-satisfaction scoring. Many studies have thus far have shown high rates of complications and poor outcomes. Spear found that PMRT was inferior to pre-recon radiation, with higher rates of failure (21% vs. 11%) (29), and other studies have found complication rates as high as 40% (30,31). Tang et al. also found that post-mastectomy radiation had higher failure rates over pMRT in a study reviewing 982 NSMs, but additionally noted that the summation of risk factors led to worse outcomes (28). In irradiated patients, complication rates were 13.4% without further risk factors, and 17.5%, 50% and 66.7% when 1, 2 and ≥3 additional independent risk factors were present, respectively (P<0.001). Nipple loss was 4% and 4.3% respectively for pMRT and PMRT compared to 0.9% in non-irradiated patients. Reisch and Tang both found that with the appropriate and careful selection of patients, however, success can be as high as 90% and radiation is therefore not a contraindication to NSM with immediate reconstruction (27,28).
At our institution, we have had 40 patients who have undergone NSM either after pMRT or undergone PMRT therapy. Our preliminary data presented this year at ASBS 2017 with half of the patients undergoing pre NSM radiation therapy and half undergoing post NSM radiation therapy. We analyzed incidence of complications, QoL, and patient satisfaction. Similar to the other studies, we found an overall higher complication rate in the PMRT cohort vs.those undergoing pMRT therapy (61.9% vs. 31.6%, P=0.067). Not surprisingly, complications requiring operative intervention were significantly higher in the PMRT cohort (38.1% vs. 5.3%, P=0.021). Ultimately nipple areolar complex survival was higher in the pMRT cohort 100% vs. PMRT 85.7%. In addition, the breast-related QoL scores were superior in the pMRT group, and the overall satisfaction scores were high in both groups. All patients that either have received pre NSM radiation or anticipate post NSM radiation therapy are informed of the higher complication rate and risk of reoperation. Despite this risk of future complications and asymmetry, patients have a high overall QoL and patient satisfaction with retaining their nipple.
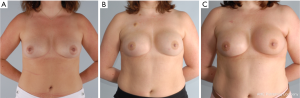
In these patients with either pre or post NSM radiation therapy, meticulous technique is paramount for preserving blood supply. This includes limiting tension on the flaps, maintaining the blood supply to the flap through the second intercostal perforator and extending the incision to limit tension on the flaps. An inframammary skin incision is recommended when possible to avoid fibrosis and asymmetry. Figure 4 demonstrates a patient who underwent NSM and immediate prepectoral reconstruction, followed by postoperative radiation with an excellent cosmetic outcome.
NSM with high BMI, ptotic breasts and previous breast reduction
Although some authors have recommend excluding NSM in patients with a high BMI [30–40 kg/m2 and/or large breast size (>500 g)] to avoid a high complication rate and poor outcomes, this criteria has significantly changed in the last five years. Careful preoperative surgical planning with both the breast surgeon and reconstructive surgeon has allowed these non-ideal patients to become potential candidates for NSM. In the study by Coopey, the mean BMI was 24.0 kg/m2 (range, 16.9–44.8 kg/m2) (17). Mean breast volume increased through the years (mean of 366 cm3 in 2007–2010, increased to 519 cm3 in 2011–2012) (17). Excellent outcomes were achieved, with nipple necrosis in only 1.7% of patients (17). Other studies have also shown similar outcomes: Filho et al. had only 1/341 (0.2%) patients with partial loss of the nipple due to ischemia (9) and Jensen et al. had 8/127 (6%) patients with nipple necrosis and subsequent removal (19).
Historically, several authors only considered NSM in the ideal candidate with small breasts and absence of ptosis with the removal of no skin. Currently, ptotic breasts are no longer a contraindication for NSM. There are several approaches to the ptotic patient depending on the degree of ptosis. Reconstructive surgeons have been instrumental in the planning of incisions that allow for excision of redundant skin at the time of NSM. Coopey describes a crescent-shaped incision superior to the areola and a vertical incision placed inferiorly from the edge of the areola to the inframammary fold (IMF), similar to a wise pattern. Dietz et al. uses a skin reduction technique, in which excess skin is de-epithelialized to preserve the dermal vessels (32). After mastectomy, the excess skin is imbricated to reduce the skin envelope. The de-epithelialized lower flap “autoderm” can be sewn to the pectoralis muscle for coverage of the tissue expander. This method allows preservation of the nipple and leads to improved cosmesis (32). A similar technique is described by Zannis at the American Society of Breast Surgeons National Meeting in 2017 as well as Folli, in which ptotic breasts are addressed by performing NSM with simultaneous mastopexy, allowing for direct to implant reconstruction (33). Folli reported a Wise pattern and bipedicle dermal flap to preserve the NAC (33,34).
Spear et al. describes a two-stage approach, in which the cancer resection with oncoplastic breast reduction is performed in the first stage, followed by a definitive NSM in approximately 8 weeks once the nipple has revascularized in its new position (35). At our institution, the use of Novadaq Spy Fluorescence Imaging technology which provides reconstructive surgeons with real-time visualization of perfusion through the use of indocyanine green injection is used intraoperatively in these complex cases at the 2nd stage NSM to assure skin and nipple areolar viability. If the viability is pristine, patients then undergo either a pre-pectoral or retropectoral direct to implant reconstruction. If the Spy shows decreased viability then a tissue expander is alternatively placed. This technique has been described and successful among breast and reconstructive surgeons at other institutions as well (34,36,37). Regarding technique, Spear suggested the best incisions are IMF, lateral, radial or lateral mammary fold (35). In our more recent experience, however, the majority of NSMs can be safely performed through the IMF, resulting in better cosmesis with decreased nipple areolar complications.
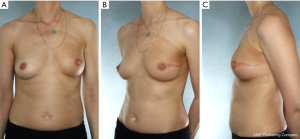
Our approach for a patient with grade 3 ptosis with BRCA genetic mutation for preventative surgery from Medstar Georgetown University Hospital includes a two-stage technique as shown below in Figure 5. She underwent an initial mastopexy with areolar reduction followed by NSM with tissue expander and implant reconstruction 8 weeks later. This patient was allergic to dye and could not undergo intraoperative Spy assessment of her flaps. Therefore, a tissue expander was placed in lite of the recent mastopexy and implant exchange was performed at a later date achieving excellent cosmesis and overall high patient satisfaction.
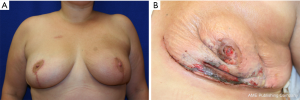
In the setting of a previous mastopexy or reduction, we do recommend placement of a tissue expander at the 2nd stage and free flap reconstruction at a later date to reduce flap swelling and tension on the overlying NSM skin envelope. Although immediate free flap reconstruction at the time of NSM without prior tissue expander placement can be successful (Figure 6), this can potentially lead to a higher incidence of flap ischemia and necrosis, especially in the inferior quadrants, as demonstrated in Figure 7. These areas are watershed areas and the most distant from the blood supply.
Nipple sparing mastectomy after neoadjuvant chemotherapy
The use of neoadjuvant chemotherapy can downsize the tumor and allow surgeons to obtain negative margins making non-ideal patients candidates for NSM. These patients should be approached carefully in close collaboration with a multidisciplinary team. Imaging with mammogram and breast MRI before and after systemic treatment is used to monitor response to treatment. Patients with a good response to systemic treatment with a definite plane between the nipple areolar complex and skin to tumor may be offered a NSM. If PMRT is anticipated, then patients are counseled that their postoperative cosmesis may be compromised with radiation related changes such as fibrosis, asymmetry and a high riding nipple, demonstrated in Figure 8.
In a study by Agresti et al., 361 patients who underwent NSM as first line therapy were compared to 61 patients who underwent primary chemotherapy followed by NSM (NSM-PC) (38).There was no significant difference in the rate of nipple-areola involvement in the NSM and NSM-PC groups [13.3% and 9.8%, respectively (P=0.539)]. NAC involvement in these two groups was significantly associated with tumor size, multicentric or multifocal tumor, and the presence of an intraductal component. An additional 151 patients who underwent PC followed by conventional total mastectomy (TM-PC) were compared to the NSM-PC group. There was no significant difference in 4-year local disease free survival (LDFS) between the NSM-PC and TM-PC groups at 0.89 (95% CI, 0.77–0.95) and 0.93 (95% CI, 0.83–0.97), respectively. LDFS was compared between the NSM and NSM-PC group using tumor size before and after chemotherapy. The hazard ratio between the two groups was comparable when using pre-chemotherapy tumor size, however, NSM-PC patients showed a significantly greater hazard of local relapse than did the NSM patients when using post-chemotherapy tumor size. This study does show however, that NSM after neoadjuvant chemotherapy is oncologically safe. Rates of local relapse were related to disease stage, and there was no significant association with the type of surgery performed.
Another study by Wengler et al. showed that immediate reconstruction in NSM after neoadjuvant chemotherapy is also safe with both early stage and advanced breast cancer (39). 280 breast cancers were treated with skin-sparing mastectomy (SSM) (94%) or NSM (6%) after neoadjuvant chemotherapy followed by immediate reconstruction with either tissue expander (83.6%), implant (1.4%), or autologous flap (15%). Thirty-day complications were at a low rate (13.2%) and significantly associated with BMI (P<0.0001), tobacco use (P=0.015), and adjuvant radiation (P=0.025). Overall PMRT therapy complications increased to 17.2%. Implant or expander loss rate before radiation was 7.1% and increased to 18.4% after PMRT (P=0.03). During the study period of 45 months, the local-regional recurrence rate was 3.2% (n=9) and distant recurrence was 13.2% (n=37). Variables predicting recurrence were pre-neoadjuvant chemotherapy tumor size, residual tumor size, and grade 3 vs. grade 2 histology, HER2 negative status, and lack of pathologic complete response. This data supports the safe use of immediate reconstruction after neoadjuvant chemotherapy followed by NSM or SSM with careful consideration into the patient’s individual risk factors and use of PMRT. Finally, a study by Frey et al. showed that it is the combination of neoadjuvant chemotherapy plus adjuvant chemotherapy that leads to the highest rate of complications with a NAC necrosis rate of 42.9% (n=3 out of 7 total) compared to neoadjuvant or adjuvant chemotherapy alone (40).
Conclusions
Several retrospective studies in the last decade have shown that NSM is oncologically safe while achieving superior cosmesis and high patient satisfaction. The surgical oncologic goal of this procedure is to achieve negative margins. Each patient should be treated in a multidisciplinary setting to determine whether the patient is both an oncologically safe and feasible reconstructive candidate. Ideal patients for NSM include patient with A or B cup breast size, BMI <30 kg/m2, no or minimal ptosis and not actively smoking. However, multiple factors can contribute to increased risk of complications and make a patient a non-ideal candidate. Patients that are at high risk of complications with poor outcomes include those who have had previous or anticipated radiation, macromastia (C cup breast size or larger), women with ptotic breasts (grade 2 or 3), high BMI (>30 kg/m2) and active smokers. Although a small number of patients may be found to have postoperative positive nipple margins or nipple necrosis, management still includes excision of the nipple areolar complex to achieve negative margins. We employ the use of fluorescence imaging technologies for high risk complex cases in order to determine flap viability and help guide the immediate reconstruction. The surgical oncologic goal is to achieve negative margins and ultimately local control. Multidisciplinary planning, new surgical and reconstructive techniques and targeted systemic treatments have enabled the inclusion criteria for NSM to significantly widen allowing more patients to benefit from this procedure.
Acknowledgements
This overview was partially supported by the Division of Breast Surgery at MedStar Georgetown University Hospital. We are thankful to our colleagues Dr. Troy Pittman, Dr. Scott Spear and Dr. John Sherman who provided patient photography and their expertise. We are also grateful to our colleague Dr. Troy Pittman for his collaboration with the surgical technique for management of the positive nipple margin.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patients displayed in the photos for permission to publish.
References
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst 2001;93:1633-7. [Crossref] [PubMed]
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 2015;22:370-6. [Crossref] [PubMed]
- Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2001;345:159-64. [Crossref] [PubMed]
- Heemskerk-Gerritsen BA, Brekelmans CT, Menke-Pluymers MB, et al. Prophylactic mastectomy in BRCA1/2 mutation carriers and women at risk of hereditary breast cancer: long-term experiences at the Rotterdam Family Cancer Clinic. Ann Surg Oncol 2007;14:3335-44. [Crossref] [PubMed]
- Peled AW, Irwin CS, Hwang ES, et al. Total skin-sparing mastectomy in BRCA mutation carriers. Ann Surg Oncol 2014;21:37-41. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967-75. [Crossref] [PubMed]
- Willey SC, Spear SL, Hammond DC, et al. Surgery of the Breast: Principles and Art-Two-Volume Set. Lippincott Williams & Wilkins, 2011.
- McDonnell SK, Schaid DJ, Myers JL, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol 2001;19:3938-43. [Crossref] [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [Crossref] [PubMed]
- Gerber B, Krause A, Reimer T, et al. Skin-sparing mastectomy with conservation of the nipple–areola complex and autologous reconstruction is an oncologically safe procedure. Annals of surgery 2003;238:120. [Crossref] [PubMed]
- Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol 2009;27:4948-54. [Crossref] [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [Crossref] [PubMed]
- Crowe JP, Patrick RJ, Yetman RJ, et al. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch Surg 2008;143:1106-10. [Crossref] [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [Crossref] [PubMed]
- Wagner JL, Fearmonti R, Hunt KK, et al. Prospective evaluation of the nipple–areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137-44. [Crossref] [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [Crossref] [PubMed]
- Tang R, Coopey SB, Merrill AL, et al. Positive nipple margins in nipple-sparing mastectomies: rates, management, and oncologic safety. J Am Coll Surg 2016;222:1149-55. [Crossref] [PubMed]
- Eisenberg RE, Chan JS, Swistel AJ, et al. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J 2014;20:15-21. [Crossref] [PubMed]
- Alperovich M, Choi M, Karp NS, et al. Nipple-sparing Mastectomy and Sub-areolar Biopsy: To Freeze or not to Freeze? Evaluating the Role of Sub-areolar Intraoperative Frozen Section. Breast J 2016;22:18-23. [Crossref] [PubMed]
- Rusby JE, Brachtel EF, Taghian A, et al. Microscopic anatomy within the nipple: implications for nipple-sparing mastectomy. Am J Surg 2007;194:433-7. [Crossref] [PubMed]
- Rusby JE, Brachtel EF, Michaelson JS, et al. Breast duct anatomy in the human nipple: three-dimensional patterns and clinical implications. Breast Cancer Res Treat 2007;106:171-9. [Crossref] [PubMed]
- Reish RG, Lin A, Phillips NA, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. Plast Reconstr Surg 2015;135:959-66. [Crossref] [PubMed]
- Tang R, Coopey SB, Colwell AS, et al. Nipple-sparing mastectomy in irradiated breasts: selecting patients to minimize complications. Ann Surg Oncol 2015;22:3331-7. [Crossref] [PubMed]
- Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 2012;130:1-9. [Crossref] [PubMed]
- Ascherman JA, Hanasono MM, Newman MI, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg 2006;117:359-65. [Crossref] [PubMed]
- Hirsch EM, Seth AK, Dumanian GA, et al. Outcomes of tissue expander/implant breast reconstruction in the setting of prereconstruction radiation. Plast Reconstr Surg 2012;129:354-61. [Crossref] [PubMed]
- Dietz J, Fedele G. Skin reduction nipple-sparing mastectomy. Ann Surg Oncol 2015;22:3404. [Crossref] [PubMed]
- Folli S, Mingozzi M, Curcio A, et al. Nipple-sparing mastectomy: an alternative technique for large ptotic breasts. J Am Coll Surg 2015;220:e65-9. [Crossref] [PubMed]
- Martinovic ME, Pellicane JV, Blanchet NP. Surgical Delay of the Nipple–Areolar Complex in High-risk Nipple-sparing Mastectomy Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e760. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Alperovich M, Tanna N, Samra F, et al. Nipple-sparing mastectomy in patients with a history of reduction mammaplasty or mastopexy: how safe is it? Plast Reconstr Surg 2013;131:962-7. [Crossref] [PubMed]
- Rodriguez-Feliz J, Codner MA. Embrace the change: incorporating single-stage implant breast reconstruction into your practice. Plast Reconstr Surg 2015;136:221-31. [Crossref] [PubMed]
- Agresti R, Sandri M, Gennaro M, et al. Evaluation of Local Oncologic Safety in Nipple–Areola Complex-sparing Mastectomy After Primary Chemotherapy: A Propensity Score-matched Study. Clin Breast Cancer 2017;17:219-31. [Crossref] [PubMed]
- Wengler CA, Valente SA, AlHilli Z, et al. Determinants of short and long term outcomes in patients undergoing immediate breast reconstruction following neoadjuvant chemotherapy. J Surg Oncol 2017;116:797-802. [Crossref] [PubMed]
- Frey JD, Choi M, Karp NS. The Effect of Neoadjuvant Chemotherapy Compared to Adjuvant Chemotherapy in Healing after Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;139:10e-9e. [Crossref] [PubMed]


