Current role of interventional radiology in the management of visceral and bone metastases from thyroid cancer
Introduction
Even though the life-time risk of developing thyroid carcinoma (TC) is moderately low [<1% in the United States (1)], its incidence has almost tripled within the last few decades. This is thought to be mostly due to the increase in the detection rate offered by high-resolution sonography. Histologically, the vast majority of TCs arise from the epithelial cells and include papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary thyroid carcinoma (MTC), anaplastic thyroid carcinoma (ATC) and Hürthle cell cancers as well as the poorly differentiated variants (2). PTC, FTC, and ATC arise from the follicular cells and MTC arises from the para-follicular cells (or C-cells).
PTC and FTC represent the vast majority (90%) of the TCs (3), express sodium iodine symporters on their cellular membrane, share the same therapeutic strategies and have similar prognoses. For these reasons, they are classified as differentiated thyroid carcinoma (DTC). The clinical features of different TCs are summarized in Table 1.

Full table
According to the American Thyroid Association (ATA) guidelines (5), DTC treatment comprises a complete or partial thyroidectomy which is generally followed by the remnant ablation using radioiodine therapy (RIT). The treatment option is based on the patients’ risk-stratification according to the combination of the ATA and TNM risk stratifications (Table 2). These strategies are generally deemed sufficient for disease control. In fact, the metastatic spread is only noted in 10% of the patients affected by DTC. These metastatic lesions are treated by RIT, tyrosine kinase inhibitors (TKIs) for RIT-refractory disease (which has a worse prognosis in comparison to the RIT-non refractory disease), external beam radiation therapy (EBRT), surgical resection of the target metastases when clinically possible and the local interventional treatments.

Full table
In this narrative review, we present the current status of interventional radiology treatments for the management of TC distant metastases with a specific focus on DTCs.
Metastatic disease
ATC is the most aggressive type of TC and has the highest prevalence of the distant metastases which is seen in about 43% of the patients at presentation (6). On the other hand, the distant metastases have been reported in up to 25% of cases of MTC (7) and 10% of patients with DTC (8). Among DTCs, PTC is more likely to spread through lymphatics, thus often resulting in micro- or macro-metastases to cervical lymph-nodes (9). The common sites of metastatic spread based on the TC histotype are reported in Table 1.
Following the treatment of the primary DTC or MTC tumor, the patients undergo an active surveillance in order to detect the local or systemic spread of the primary TC as early as possible. Typical monitoring programs include a combination of tumor marker measurements [thyroglobulin (Tg) for DTC, calcitonin for MTC] and imaging follow-up (i.e., diagnostic whole-body radioiodine scans, 18FDG-PET, CT, MRI). Based on the primary TC histotype and the site of disease relapse, different therapeutic strategies are adopted (5,10).
Rationale for interventional treatments
According to the ATA guidelines (5), various therapeutic options can be considered for the management of the TC metastatic disease. This includes direct image-guided local treatments. In particular, local treatments are preferentially opted due to the following reasons:
- Whilst overall morbidity and mortality increase in patients with distant metastases, the individual prognosis can vary based on the various inherent clinical characteristics of these pathologies (e.g., age at diagnosis of metastases, distribution and number of metastases, histology of the primary tumor and 18FDG as well as the radioiodine avidity);
- Improved survival is directly associated with the responsiveness to directed therapy (surgery, EBRT, thermal ablation, etc.) and/or RIT;
- Significant palliation and reduced morbidity can be achieved by the direct local treatments;
- Mutation profiling of metastatic tumor to detect genetic abnormalities (e.g., BRAF, TERT, RAS, or PAX8/PPARc) has not yet conclusively proven to be of significant value to predict the prognosis or response to the novel treatments such as TKIs. As such, TKIs are currently primarily applied in the setting of dedicated research trials and often in the tertiary referral centers;
- There is little or no benefit when RIT is applied in RIT-refractory DTCs.
The ATA guidelines (5) recommend local treatments including those performed by interventional radiologists mainly in two clinical scenarios:
- Prior to the systemic treatments when a distant metastasis is symptomatic or at high risk of local complication;
- In case of a solitary metastatic lesion exhibiting an evolution despite the systemic therapy.
Nevertheless, due to the rarity, complexity and the diversity of the clinical scenarios, the therapeutic decision-making for the metastatic TC disease is generally tailored on an individual per-case basis by the multi-disciplinary tumor boards within the tertiary referral centres. The general rule followed while proposing local treatments is whether they are intended to provide palliation or radical local tumor control (i.e., curative treatments) in the cases of oligo-metastatic disease (11). As such, several different factors (11) are taken into consideration in order to select the best local treatment. These include:
- The number and location of the target metastatic lesion(s); in particular, whether a solitary bone involvement is present as it has a better overall prognosis when compared to a visceral involvement (12);
- Whether the metastases are symptomatic;
- Whether metastases are RIT-sensitive and if so, whether RIT has been applied to the maximum permissible dose (i.e., 600 mCi);
- Whether the metastases demonstrate 18FDG uptake thus, indicating a more aggressive tumour histotype;
- The patients’ life expectancy;
- The progression rate of the disease (i.e., slow evolving disease are more likely to receive the curative rather than the palliative treatments);
- The local availability of physicians performing local treatments.
Interventional techniques and available results
The percutaneous interventional techniques for the treatment of the TC distant metastases are categorized into the vascular, ablative and consolidative treatments.
The vascular techniques are essentially in the form of trans-arterial embolization (TAE) and exploit the hyper-vascular characteristic of the TC visceral or bone metastases.
The ablative techniques are applied to achieve partial or complete necrosis of the neoplastic tissue by causing a dramatic increase [≥55 °C in radiofrequency (RFA) or microwave ablation (MWA)] or decrease [around −40 °C through cryo-ablation (CA)] of the intra-tumoral temperature (13) in the visceral or bone metastases.
Finally, the consolidative techniques are specifically applied for the treatment of the lytic bone metastases in order to prevent or fix a pathologic fracture. Their aim is to obtain the reinforcement of bone defect through percutaneous injection of polymethylmethacrylate (PMMA) cement (i.e., cementoplasty or osteoplasty). Depending on the biomechanics of the involved bone, surgical or non-surgical bone reinforcement techniques (e.g., endomedullary nailing, percutaneous screw fixation, etc.) may be combined with the cementoplasty to increase the bone reinforcement (14-18).
All the aforementioned treatments can be combined and tailored according to the specific needs of the patient.
Visceral metastases
Ablation
Lung and liver are the most common sites of the visceral metastases in patients with TC. Unfortunately, to the best of our knowledge, specific series addressing interventional treatments in such scenarios are substantially lacking (19,20). Nevertheless, a large French series reported by de Baère et al. about the RFA of lung metastases from different primary tumors such as colorectal (52%), renal (12%), soft tissue (9%) or TC (3%), revealed that RFA results are comparable to those reported by the surgical series reporting the outcome of the lung metastasectomy (20). The series included 566 patients (mean age, 62.7±13.2 years old). A total of 1,037 lung metastases (median diameter, 15 mm; range, 4–70 mm) were treated. The median follow-up was 35.5 months and the median overall survival (OS) was 62 months. Four-year local efficacy was reported to be 89%. Four-year lung disease control rate was 44.1%, with the patients being re-treated safely for up to four times. Among the 1,037 treated metastases, there were 86 local progressions with the rates of local tumor progression per tumor of 5.9%, 8.5%, 10.2%, and 11.0% at 1, 2, 3 and 4 years, respectively. Size of tumor predicted local tumor progression. The rectal primary tumors, metastatic lesions >2 cm, high number of metastases and the extra-pulmonary involvement were associated with the lower progression-free survival. The sub-population of patients presenting with colorectal cancer metastases, size >2 cm, number of metastases ≥3, rectal origin of the primary tumor and the extra-pulmonary disease was significantly or borderline significantly associated with the lower progression-free survival.
Primary origin, disease-free interval, size and number of metastases were associated with OS in multivariate analysis. Progression at RFA site was associated with poor OS. In the 293 colorectal cancer metastases, size >2 cm and a number of metastases ≥3 remained significantly associated with the reduced OS.
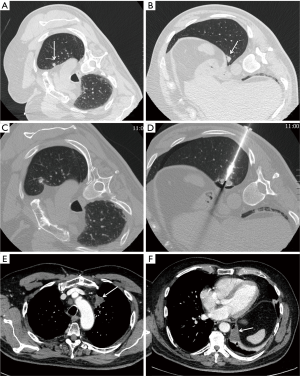
Authors concluded that RFA should be considered as an option for treatment of small size lung metastases, namely the ones below 2–3 cm. Therefore, in the setting of lung metastases from TC, it is reasonable to consider RFA with a curative intent in the patients with slow-evolving, macro-nodular disease, sized <3 cm (11) (Figure 1). On the other hand, miliary disease should be considered as a contraindication to ablation.
A large American series about RFA of 233 colorectal cancer liver metastases in 162 patients reported a median local tumor progression-free survival of 26 months (21). At univariate analysis, predictors of a shorter local tumor progression-free survival were: the tumor size >3 cm, ablation margin size ≤5 mm, high modified clinical risk score [made-up of nodal status of the primary tumor, disease-free interval from primary tumor resection to liver metastasis detection, number of liver tumors, size of the largest tumor (cut-off 3 cm) and carcinoembryonic antigen (CEA) level (cut-off 30 ng/mL)], male sex and no history of prior hepatectomy or hepatic arterial infusion chemotherapy. The multivariate analysis demonstrated that only tumor size >3 cm and margin size ≤5 mm were independently associated with a shorter local tumor progression-free survival. The median OS and 5-year OS were 36 months and 31% respectively. The univariate analysis demonstrated that the predictors of a shorter OS were: tumor size >3 cm, CEA level >30 ng/mL, high modified clinical risk score and extra-hepatic disease. At multivariate analysis, tumor size >3 cm and more than one site of extra-hepatic disease were independent predictors of a shorter OS.
Similar to the hepatic metastases of the colorectal cancer, liver metastases from TC can be reasonably treated with curative RFA providing that the tumor size <3 cm coupled to large margins of ablation (11).
Embolization
Different interventional teams have published small series regarding the embolization of liver metastases from MTC (22-24). One of the largest published series (23) applied lipiodol-doxorubicin TAE for the treatment of MTC patients with predominant and progressive liver metastases. In this series, 12 patients underwent 18 interventional procedures. Partial radiological tumor response was achieved in five patients (42%) with a median duration of 17 months, stabilization in 5 (42%) with a median duration of 24 months and finally the tumor progression was seen in the remaining 2 (16%). The 5 partial tumor responses were observed in the nine patients with <30% liver involvement. Clinical response (i.e., >25% decrease of symptoms intensity) was observed in two of the five patients with diarrhoea. One major tumor necrosis was noted after TAE. Authors concluded that lipiodol-doxorubicin TAE should be considered for treating MTC patients with a progressive and predominant liver disease and preferably at an early stage during the course of metastatic disease.
Bone metastases
Bone metastases from TC can be managed with all the available techniques (i.e., vascular, ablative and consolidative) (16). These techniques can be applied alone or in combination in accordance with the final therapeutic goal (i.e., curative vs. palliative) (25).
Embolization
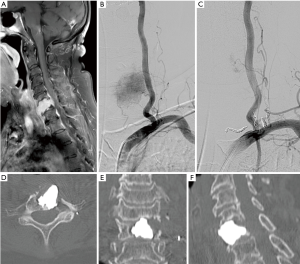
Percutaneous TAE has been largely applied for the treatment of the bone metastases from TC either alone (26) or in combination with the other treatments (27) (Figure 2) including RIT (28). Better results in terms of symptoms control and serum Tg decrease were noted when TAE was combined with the other treatment modalities such as EBRT, surgery and RIT (27); as such, the reported serum Tg levels demonstrate a more substantial reduction of tumor burden in such scenarios (27,28).
Ablation and consolidation
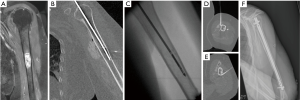
Following bone ablation, consolidation with surgical or percutaneous techniques is mandatory in bones subjected to mechanical stresses, in order to prevent secondary bone insufficiency fractures (Figure 3).
Cazzato et al. (16) have published their experience with interventional management of bone metastases from TC in 25 patients (median age, 61.1 years). In this series, a total of 54 metastases (mean maximum diameter, 57.4±36.4 mm) were treated with cementoplasty (77.55%), CA (14.29%) and RFA (8.16%). Mean RIT dose administered before the intervention was 276.6±270.4 mCi. Four lesions (7.4%) were asymptomatic; the remaining 50 (92.6%) were symptomatic (31.5% still following EBRT and 42.6% during RIT). Forty-two lesions received additional EBRT.
Lesions were located in the spine (29.62%), pelvis (46.32%), limbs (5.55%) or in other districts (16.66%). Most of the target metastases (62.96%) were in weight-bearing bones.
The delay between the diagnosis of TC and the first interventional session was 5.4±6.2 years (median, 2.7). Most interventions were carried out under the general anaesthesia (71.4%). Mean hospitalization time required for the intervention was 2.0±1.9 days (median, 2.0). Median follow-up following the intervention was 4.6 years and complete treatment was noted in 55.6% treated metastases. No fractures, neither injuries to the nervous system were noted. One major and one minor complication were reported. OS after interventions was 71.6%, 66.8% and 60.1% at 1, 2 and 3 years, respectively. Patients presenting with only metastatic bone involvement at diagnosis showed a better OS compared to those affected by multi-organ involvement. Moreover, patients presenting with sole metastatic bone involvement at the time of the first intervention had a better OS if they presented with a solitary metastasis compared to those showing multiple metastases.
Song et al. (29) retrospectively evaluated the effectiveness of osteoplasty combined with RIT for the treatment of the bone metastases from DTC in eight patients. All patients received total thyroidectomy and oral RIT (100 mCi). Thereafter, osteoplasty was performed at 2–3 months followed by 2–5 sessions of RIT every 4–6 months after the first RIT. Following osteoplasty, the mean serum Tg decreased by 86.0%; and it further declined by 67.4% after repeated RIT. All the patients experienced pain and neurologic symptoms amelioration. There was no report of any severe complications.
Conclusions
The metastatic disease from the TC represents a complex clinical scenario, which mandates a case-based multi-disciplinary approach in tertiary referral centers. In this setting, direct localised treatments such as minimally invasive interventional radiology procedures can play a vital role in providing a timely palliative or curative treatment in accordance with the patients’ clinical status. However, due to the infrequency and pleomorphic characteristic of the TC metastatic disease, the dedicated series reporting the outcome of the interventional treatments of the metastatic TC are few, retrospective and often without a prolonged follow-up. Therefore, dedicated prospective series are desirable to understand the exact role of the minimally invasive interventional therapies. As such, a particular attention should be paid to the ablative therapies with curative intent for the metastatic lesions in the oligo-metastatic TC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- NCCN Clinical Practice Guidelines in Oncology Thyroid Carcinoma Version 2.2015. Available online: Published July 29, 2015. Accessed November 28, 2015.http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
- De Lellis RA. World Health Organization classification of tumours: pathology and genetics of tumours of endocrine organs. Lyon, France: IARC, 2004.
- Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11. [Crossref] [PubMed]
- Kelil T, Keraliya AR, Howard SA, et al. Current Concepts in the Molecular Genetics and Management of Thyroid Cancer: An Update for Radiologists. Radiographics 2016;36:1478-93. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma: treatment outcome and prognostic factors. Cancer 2005;103:1330-5. [Crossref] [PubMed]
- Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma: a population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 1997;79:564-73. [Crossref] [PubMed]
- Schmidbauer B, Menhart K, Hellwig D, et al. Differentiated Thyroid Cancer—Treatment: State of the Art. Int J Mol Sci 2017;18:1292. [Crossref] [PubMed]
- Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg 2000;24:942-51. [Crossref] [PubMed]
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet 2016;388:2783-95. [Crossref] [PubMed]
- Bonichon F, Buy X, Godbert Y, et al. Traitement local des métastases de cancer thyroïdien différencié. Ann Endocrinol 2015;76:1S40-6.
- Cazzato RL, Bonichon F, Buy X, et al. Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer. Eur J Surg Oncol 2015;41:1247-55. [Crossref] [PubMed]
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [Crossref] [PubMed]
- Kim Y, Guy H, Sung T, et al. Palliative percutaneous stabilization of lower extremity for bone metastasis using fl exible nails and bone cement. Surg Oncol 2014;23:192-8. [Crossref] [PubMed]
- Garnon J, Koch G, Ramamurthy N, et al. Percutaneous CT and Fluoroscopy-Guided Screw Fixation of Pathological Fractures in the Shoulder Girdle: Technical Report of 3 Cases. Cardiovasc Intervent Radiol 2016;39:1332-8. [Crossref] [PubMed]
- Cazzato RL, Buy X, Grasso RF, et al. Interventional Radiologist’s perspective on the management of bone metastatic disease. Eur J Surg Oncol 2015;41:967-74. [Crossref] [PubMed]
- Cazzato RL, Garnon J, Tsoumakidou G, et al. Percutaneous image-guided screws meditated osteosynthesis of impeding and pathological/insufficiency fractures of the femoral neck in non-surgical cancer patients. Eur J Radiol 2017;90:1-5. [Crossref] [PubMed]
- Cazzato RL, Koch G, Buy X, et al. Percutaneous Image-Guided Screw Fixation of Bone Lesions in Cancer Patients: Double-Centre Analysis of Outcomes including Local Evolution of the Treated Focus. Cardiovasc Intervent Radiol 2016;39:1455-63. [Crossref] [PubMed]
- Wertenbroek MW, Links TP, Prins TR, et al. Radiofrequency ablation of hepatic metastases from thyroid carcinoma. Thyroid 2008;18:1105-10. [Crossref] [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [Crossref] [PubMed]
- Shady W, Petre EN, Gonen M, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes--A 10-year Experience at a Single Center. Radiology 2016;278:601-11. [Crossref] [PubMed]
- Lorenz K, Brauckhoff M, Behrmann C, et al. Selective arterial chemoembolization for hepatic metastases from medullary thyroid carcinoma. Surgery 2005;138:986-93; discussion 993. [Crossref] [PubMed]
- Fromigué J, De Baere T, Baudin E. Chemoembolization for liver metastases from medullary thyroid carcinoma. J Clin Endocrinol Metab 2006;91:2496-9. [Crossref] [PubMed]
- Grozinsky-Glasberg S, Bloom AI, Lev-Cohain N, et al. The role of hepatic trans-arterial chemoembolization in metastatic medullary thyroid carcinoma: a specialist center experience and review of the literature. Eur J Endocrinol 2017;176:461-8. [Crossref] [PubMed]
- Gangi A, Tsoumakidou G, Buy X, et al. Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol 2010;33:706-13. [Crossref] [PubMed]
- De Vries MM, Persoon AC, Jager PL, et al. Embolization therapy of bone metastases from epithelial thyroid carcinoma: effect on symptoms and serum thyroglobulin. Thyroid 2008;18:1277-84. [Crossref] [PubMed]
- Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184-9. [Crossref] [PubMed]
- Van Tol KM, Hew JM, Jager PL, et al. Embolization in combination with radioiodine therapy for bone metastases from differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2000;52:653-9. [Crossref] [PubMed]
- Song HJ, Wu CG, Xue YL, et al. Percutaneous osteoplasty combined with radioiodine therapy as a treatment for bone metastasis developing after differentiated thyroid carcinoma. Clin Nucl Med 2012;37:e129-33. [Crossref] [PubMed]


