Allo- and auto-percutaneous intra-portal pancreatic islet transplantation (PIPIT) for diabetes cure and prevention: the role of imaging and interventional radiology
IntroductionOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
In the United Kingdom 10% of the National Health Service budget is spent on diabetes, mainly due to diabetic-induced heart, foot, and renal diseases (1).
The acute life-threatening and long-term complications from type 1 diabetes mellitus (T1DM) are significant causes of both mortality and morbidity (2,3).
Good glycemic control with intensive insulin treatment is known to markedly decrease the incidence of chronic micro-vascular complications and cardiovascular morbidity in patients with T1DM (2,4). However, this treatment is difficult, expensive, and associated with an increased incidence of severe hypoglycemia, which is often accompanied by hypoglycemic unawareness (5), provoking considerable complications (6).
Whole-organ pancreatic transplants into human subjects first performed in the early 1980s (7) are now associated with 1-year insulin independence rates of higher than 80% (8). Long-term T1DM is often associated with chronic renal insufficiency: a combined kidney/pancreas transplantation represented the best therapeutic option for these patients (9,10), normalizing glucose levels and preventing complications (11,12). However, the procedure is also associated with significant perioperative morbidity (11) and the need for long life immunosuppression therapy.
Since the 1990s, islet-after-kidney (IAK) transplantation represents a good alternative to treat diabetes associated with chronic renal insufficiency (13) in case of pancreas unavailability from a cadaveric donor at the time of kidney transplantation. Since the 2000s, according to the Edmonton Protocol (14) based on the infusion of a large islet mass and on a glucocorticoid-free immunosuppressive regimen, islet-transplant-alone (ITA) is performed in patients affected by brittle T1DM with preserved renal function: the preliminary results, published by Shapiro et al. (14) demonstrating an 80% of insulin independence rate at 1 year, paved the way for further experiments worldwide. However in both diabetic populations submitted to allo-PIPIT (IAK an ITA), the purpose is to obtain insulin independence or a significant reduction of exogenous insulin requirement, to prevent hypoglycaemic episodes and diabetic complications (15,16) such as nephropathy (17) or retinopathy (18) and to increase life expectancy (19).
Auto-PIPIT has been more recently introduced not to cure but to prevent another type of diabetes known as “pancreatogenic diabetes”: it originates from an extreme disruption of glucose homeostasis after extensive pancreatic resection such as total/subtotal pancreatectomy for chronic pancreatitis or tumours (20,21). The percentage of patients undergoing pancreatectomy that develop pancreatogenic diabetes varies from 8% to 23% increasing up to 40–50% during the follow-up (22, 23). Auto-PIPIT is usually performed 12–48 h after surgery, does not require immunosuppression and has a lower rejection rate than allo-PIPIT (24).
After isolation, centrifugation and purification (10) of the islets, the technical procedure of allo- and auto-PIPIT is similar. In our center, allo- and auto-PIPIT are performed using a combined ultrasonographic and fluoroscopic guidance to reduce puncture attempts, procedural time and peri-procedural complications (25).
Imaging and interventional radiology play a crucial role in PIPIT. In the present article we present a review based on our experience started in the early 1990s and focused on radiological and interventional aspects of PIPIT: pre-PIPIT imaging, interventional procedure, early post-PIPIT imaging, and late post-PIPIT imaging will be analyzed, also highlighting the three islet-transplanted populations (IAK, ITA and auto-transplanted patients).
Criteria of analysisOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
A systematic literature review was performed to investigate the role of imaging and interventional radiology in PIPIT from 1982 to 2017. Studies regarding allo-transplantation for type 1 diabetes treatment and auto-transplantation for diabetes prevention after extensive pancreatectomy were selected. Our experience was compared with the published literature focusing on the crucial role of imaging and interventional radiology in all the phases of PIPIT: pre-PIPIT imaging, interventional procedure, early post-PIPIT imaging, and late post-PIPIT imaging. Shared and different interpretations were discussed and analyzed.
Pre-PIPIT imagingOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
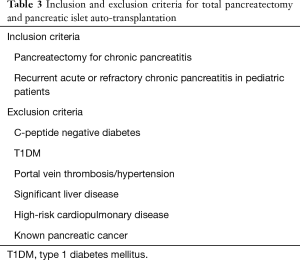
Prior to PIPIT, all patients undergo clinical, biochemical, and radiological evaluation to determine whether they meet the inclusion/exclusion criteria for allo- (Tables 1,2) and auto-transplantation (Tables 3,4). C-peptide is a unique and independent marker of insulin biosynthesis and secretion. In patients with T1DM, C-peptide negativity is used to confirm the type 1 status, and therefore, undetectable C-peptide is important in the inclusion criteria for allo-PIPIT.

Full table

Full table
Full table
Full table
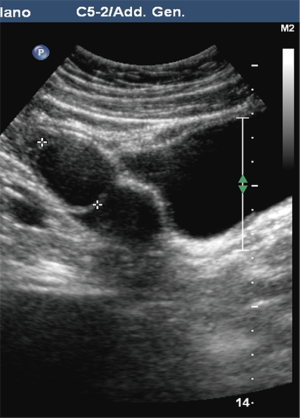
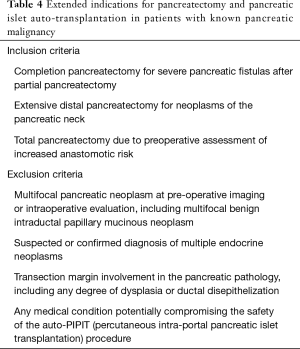
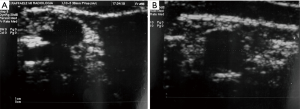
Pre-PIPIT imaging consists of chest radiography and liver color Doppler ultrasound (CDU) examination. Portal vein course, patency and flow direction are evaluated with CDU (Figure 1) to exclude vascular abnormalities. Liver echotexture is also accurately assessed to verify eventual structural changes after PIPIT.
In case of auto-PIPIT, usually performed 12–48 hours after extended pancreatectomy, CDU is also performed to exclude liquid or hemorrhagic post-surgical collections; in some circumstances a contrast-enhanced computed tomography (CT) may be required.
Interventional procedureOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
Patient preparation
PIPIT is usually performed in an angiographic suite under sterile condition with intravenous moderate sedation and routine hemodynamic, cardiac, and oxygen-saturation monitoring (26).
The portal venous system is the anatomic site of choice for islet transplantation, and has advantages of minimally invasive accessibility with avoidance of systemic hyperinsulinemia (27). Although there are few published data on the use of antibiotics in islet cell transplantation, patients typically undergo peri-procedural antimicrobial and antiviral therapy.
Bacteremia and/or sepsis after PIPIT are rare, but sporadic cases related to contamination of cryopreserved islets have been reported (28).
Portal vein access and catheterization
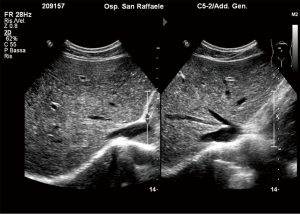
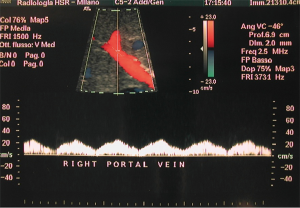
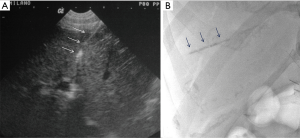
The procedure is usually performed using a combined CDU- and fluoroscopy-guided (25,29) technique. A peripheral portal vein branch is ultrasonically punctured using a right-sided intercostal approach with a 22-gauge needle (Figure 2).
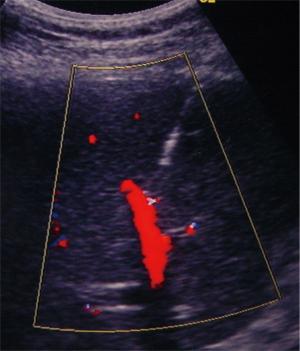
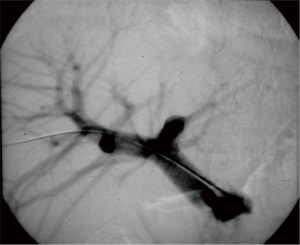
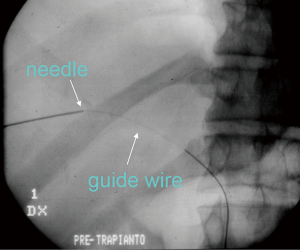
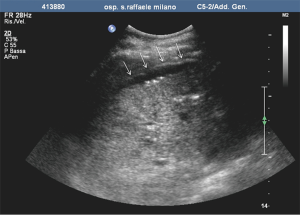
The role of ultrasound guidance is essential in order to minimize liver punctures number, procedural time and accidental puncture of other structures (arteries, hepatic veins, and gallbladder). In our center, usually the same operator, experienced in both CDU and interventional radiology, performs the right portal vein puncture by inserting the needle with the right hand while holding the probe in the left hand to ultrasonically guide the puncture (25). After the puncture of one wall of a branch of the right portal vein, fluoroscopic guidance is used to perform main trunk portal vein catheterization advancing a 0.018-inch guidewire (Figure 3) and then a straight end-hole 4 Fr catheter over the guidewire. Portography (Figure 4) and portal venous pressure measurement are performed before and after islets infusion to verify portal patency and pressure increase after PIPIT. Usually portal vein pressure measured prior to infusion is variable from 6 to 12 mmHg and increases by 1–2 mmHg after islets infusion.
Anticoagulation is necessary to reduce the risk of acute portal vein thrombosis. Our institution protocol is based on 1,500–2,000 IU of heparin infused into the portal vein with the islet suspension and 6,000 IU/d of enoxaparin administered subcutaneously for 7 days after the procedure (25). An alternative method reported is based on a systemic anticoagulation with heparin, started after portal vein catheterization, and with a dose of 5,000 U administered during the procedure (26).
Islet infusion
Islet infusion protocol depends on the particular national or institutional protocol used. A typical protocol is based on at least 10,000 islet equivalents per kilogram of body weight. An islet equivalent refers to an average standard diameter of 150 µm, as previously described by Ricordi et al. (30). Harvested islets are infused using gravity flow or direct syringe injection (31). The total infusion time is typically 20–30 minutes. A slow injection of the islets is important to avoid their catheter-mediated mechanical damage.
Gravity infusion method allows a better control of islet administration rate allowing gradual reduction of flow preventing any precipitous pressure increases (32). A baseline portal venous pressure over 20 mmHg is a contraindication to transplantation because of increased risk for portal venous thrombosis (31-33). Increased portal pressure indicates embolic saturation of the portal venous system, and increases the risk for thrombotic complications (31).
Tract embolization
Nowadays intrahepatic tract embolization is routinely performed in many centers to minimize the hemorrhagic risk. After completion of final portography, the catheter is retracted into the hepatic parenchymal tract using a combination of US and fluoroscopic guidance. Contrast medium is injected via the catheter to confirm tip position within the hepatic parenchyma before tract embolization. Embolization of the hepatic parenchymal tract can be performed using several hemostatic agents (34,35), in our center with gelfoam torpedoes (Figure 5). Care is taken to ensure deployment of the embolic material exclusively within the liver parenchymal tract to avoid intravascular embolization. The ideal embolization material seals the intrahepatic tract, is ultrasonically or fluoroscopically visible, is bioabsorbable, and does not interfere with subsequent radiological or interventional procedures (36).
Early post-PIPIT imagingOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
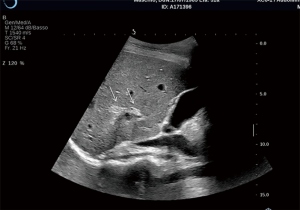
Immediate post-PIPIT imaging is based on an accurate, ultrasonographic evaluation in real time of eventual fluid collections around the liver. A strict monitoring with CDU is performed with the patient still lying on the angiographic bed in case of bleeding signs. An early bleeding diagnosis is very important to anticipate possible actions (blood drawings) and therapeutic solutions (blood transfusions, angiographic embolizations, surgical treatments). CDU of the liver is routinely performed at 1, 3 and 7 days. The two most common complications are bleeding (Figure 6) and thrombosis (Figure 7): bleeding is reported in variable percentage (about 11%), while portal vein thrombosis is more rare and reported in about 3% of the cases (37). The bleeding risk is reduced by an accurate tract embolization during catheter removal. Portal vein thrombosis represents the second most common complication after PIPIT. Complete thrombosis is very rare and a partial thrombosis usually does not determine clinical consequences. Anticoagulation therapy, based on intravenous and intraportal heparin, is routinely administered to minimize this risk. Probably thrombosis may be related to the volume, the purity, and the thrombogenicity of the infused islets (36). Other rare peri-procedural complications include arteriovenous fistulas, hemothorax, trauma to adjacent structures such as biliary tracts and gallbladder. When CDU is not diagnostic a second level imaging examination, such as contrast-enhanced CT is performed.
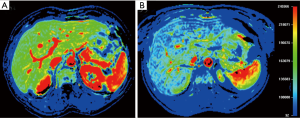
Recently, different imaging approaches were developed to directly evaluate transplanted islets viability over time. For example, pancreatic islets labeled with superparamagnetic iron oxide (SPIO) agent were detected at T2-weighted MRI sequences as dark spots scattered in the liver (Figure 8). The complete disappearance of all dark spots over time was associated with graft failure (38). Moreover, dynamic contrast enhanced (DCE) MRI was used to assess intrahepatic islet engraftment (Figure 9): early perfusion modification resulted predictive of long term function in small groups of patients (39). Several positron emission tomography (PET) tracers were explored in order to quantify liver islet engraftment. Many investigators evaluated biomarkers specific for pancreatic beta cells, with promising but not conclusive results (40). Therefore, the accurate and noninvasive detection of grafted islets remains a challenging goal.
Late post-PIPIT imagingOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
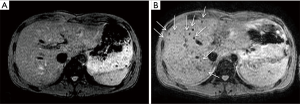
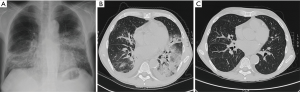
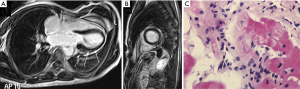
Imaging is also routinely used for PIPIT monitoring. Imaging can be used to monitor late effects due to the infused islets or the immunosuppressive treatment. Life-long immunosuppressive treatment, one of the main limitations of allo-PIPIT especially in IAK patients, can determine neoplastic and infectious complications (41). For example cytomegalovirus infections such as pneumonitis or myocarditis can likely occur after immunosuppression and impact on graft function (41), and can be detected by CT (Figure 10) and MRI (Figure 11), respectively. Following improved immunosuppressive strategies according to the Edmonton protocol, sirolimus (or tacrolimus) is routinely administered in ITA patients. Sirolimus may be associated to development of ovarian cysts (Figure 12) and to nephrotoxic effect: in patients with preserved renal function before ITA, a perinephric edema may appear at US (Figure 13) as a rim of anechoic fluid around the kidneys (42).
Immunosuppression-related complications are obviously absent in auto-transplanted patients, who are either donors or recipients and do not need immunosuppressive treatment.
Late effects and structural changes within the liver due to the infused islets can be observed from 6 to 12 months after PIPIT. Hepatic steatosis after PIPIT is determined by functioning islets which cause local insulin production, lipogenesis stimulation, and lipolysis inhibition with consequent fat development (43). Hepatic steatosis is related to liver islet engraftment, but curiously not all patients with good islet function develop imaging detectable steatosis. The relationship between graft function and steatosis appearance remains debated. Since the beginning of the 2000s steatosis detection at MRI (44,45) and US (46) after allo-PIPIT was investigated, but no univocal conclusion concerning the best technique nor any correlation between steatosis and graft function was obtained. Even in recent studies steatosis was detected in a variable percentage of patients ranging from 20% to 60% after allo-PIPIT at MRI and US (47,48) and also after auto-PIPIT at US (49): also in these prospective studies a correlation between steatosis and graft function was not clearly defined.
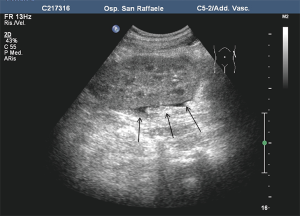
In our recent longitudinal study on 108 patients based on US (50) and involving all the 3 islet-transplanted populations (IAK, ITA and auto-transplanted patients), steatosis at US after PIPIT (Figure 14) was interpreted as an early sign of graft dysfunction (51). Our hypothesis is that steatosis appearance at US is related to the overworking activity of some residual vital stressed islets, resulting in insulin overproduction supporting other non-functioning islets: in our opinion, steatosis becomes ultrasonically detectable only when an abnormal peak of local insulin secretion is achieved (50,51). After steatosis detection at US, progressive graft exhaustion was observed until steatosis disappearance in all the patients.
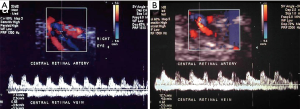
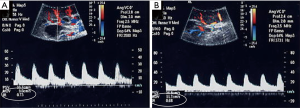
Imaging has been largely used to monitor not only the structural changes within the liver but also the beneficial effects of the pancreatic islets in different vascular districts. This has been largely important to confirm that allo-PIPIT may stabilize and reverse diabetic complications. Indeed successful islet transplantation, restoring a good glycometabolic control, improves the overall survival, the cardiovascular outcome and endothelial function in type 1 diabetic patients (19). A reversibility of endothelial dysfunction (52), typically present in type 1 diabetic patients, and an improvement of vasodilatory ability (Figure 15) was also associated with a better wellness of endothelial progenitors cells, which appeared to be less apoptotic after a successful islet transplantation (53). Imaging can provide a strong support to clinical data and highlights the benefits obtained after PIPIT. For example, a protective role on diabetic retinopathy can be supposed: a significant improvement of retinal microcirculation revealed by central retinal artery and vein flow velocity increase was found at color Doppler imaging (Figure 16) after ITA (18). On the contrary, no retinal microcirculation improvement was found in long-term type 1 diabetic patients affected by nephropathy and retinopathy, even if successfully submitted to kidney-pancreas transplantation and become insulin-independent (54): probably diabetic retinopathy may be prevented or slowed down by islet (or pancreas) transplantation only at an early stage of T1DM. The protective role of islet transplantation was also demonstrated on the renal blood flow in the transplanted kidney of IAK patients, assessing the arterial resistive index (Figure 17) at color Doppler imaging: an enhanced kidney graft survival, hypertrophy and vascular function was found after a successful islet co-transplantation (55,56).
CDU analyzing carotid intima media thickness was also used to demonstrate the beneficial effects of islet transplantation in cardiovascular function (57).
DiscussionOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
PIPIT is an innovative and valid clinical strategy to treat patients affected by brittle T1DM and to prevent pancreatogenic diabetes in patients submitted to extended pancreatectomy. To date, over 1,500 patients have undergone islet transplantation in about 40 international centers (10). Pancreatic islets are extracted, centrifuged, purified and injected via portal vein in the recipients: infused islets engraft at the level of the hepatic sinusoids to release insulin and to restore endogenous C-peptide secretion (58) The liver was deemed the most appropriate site for PIPIT due to many reasons: high regenerative capacity, double vascularization, immunological protection (59). Alternative sites for PIPIT (bone marrow, kidney capsule, gastric submucosa, genitourinary tract, omentum, testis, thymus, anterior chamber eye) were recently investigated (60) but without promising results (61). In case of allo-PIPIT, either in IAK or in ITA patients, pancreatic islets are extracted from cadaveric donors; while in case of auto-PIPIT patients are simultaneously donors and recipients. In allo-PIPIT patients need a life-long immunosuppressive treatment, while they don’t need in auto-PIPIT. Functioning islets represent an effective β-cell replacement therapy able to normalize metabolic and glycaemic control. The most important islet transplantation centers report an insulin independence rate of 50–70% by 5 years after allo-PIPIT (10,62-64), according to the Edmonton protocol. Although insulin independence is the ideal outcome from islet transplantation, it is usually not the primary endpoint. Reduction of severe hypoglycemic events and diabetic complications are other important measures of transplant outcomes (65), that can be obtained also in case of partial islet function considering also that PIPIT is a repeatable procedure. The largest series of auto-PIPIT for chronic pancreatitis report insulin independence in 15–41% of patients (66-71). The University of Minnesota reported that 30% of 409 patients submitted to auto-PIPT were insulin independent at 3 years follow up and an additional 33% had partial graft function defined by the presence of C-peptide (67).
Imaging and interventional radiology play a crucial role in PIPIT. In our experience CDU is largely used in all the phases of an islet transplant: as pre-transplant imaging to evaluate portal vein patency, as guidance in the interventional procedure to safely and quickly access the portal vein, as early post-transplant imaging to diagnose eventual complications, and as late post-transplant imaging to assess liver structural changes and vascular modifications. CDU as pre-PIPIT imaging can provide hemodynamic information about the portal vein blood flow (patency or thrombosis, flow direction, anatomic variants). In our center (and nowadays in many others) CDU is routinely used as guidance to interventional procedures and to safely access in real time the portal vein. The portal vein visibility at CDU may be slightly reduced in auto-PIPIT, performed 12–36 hours after a surgical treatment. Combined use of US and fluoroscopy for percutaneous portal venous access is associated with a low risk of complications and shorter procedure time compared with fluoroscopy alone (25,29). A combined CT and fluoroscopy approach was also used for PIPIT (72): but a very prolonged procedural time (the patient needs to be transferred to the interventional suite following the CT-guided puncture) and an increased radiation dose are significant disadvantages. Although a right or left transhepatic puncture may be used, most series report the use of a right transhepatic approach for PIPIT (29,73). CDU as early post-PIPIT imaging is important to early diagnose peri-procedural complications as bleeding and portal vein thrombosis, the two main complications of PIPIT: an early diagnosis is important to anticipate eventual therapeutic solutions (transfusions vs. anticoagulation therapy). Theoretically IAK patients affected by long term T1DM and chronically submitted to aspirin administration, should have a higher bleeding risk than ITA (and auto-transplanted) patients. In our experience, no statistically significant differences in terms of bleeding were found between IAK and ITA patients. A slightly higher bleeding rate was found in allo-transplanted patients submitted to a third PIPIT procedure than those submitted to second or first transplant (18): probably the higher portal vein pressure usually recorded during a third transplant procedure can determine a greater bleeding risk (74). An accurate intrahepatic tract embolization at the end of the procedure under ultrasonographic and/or fluoroscopic guidance can contribute to reduce the bleeding risk (74). CDU is also used as late post-PIPIT imaging to monitor liver structural changes: the appearance of steatosis after 6 months has been universally detected by US and MRI (46) but curiously not in all patients and without a shared relationship with the islet function. In our opinion, steatosis represents an early marker of islet dysfunction (51). Our hypothesis is strengthened by a recent prospective study involving all the 3 islet-transplanted populations (50). As expected, auto-transplanted patients, not needing immunosuppression, achieved better clinical outcomes than allo-transplanted patients but the percentage of steatosis ultrasonically detectable was significantly lower (4% vs. 24%). The relevant advantage of detecting steatosis at US is to early identify patients still maintaining good values of islet function (C-peptide, β score) but evolving towards graft exhaustion: these patients might receive additional immunosuppressive treatment, insulin therapy or might be listed for a further PIPIT before complete graft failure. CDU is also used to monitor vascular modifications after PIPIT. In case of successful islet transplantation, not only in case of insulin independence but also of partial islet function, restoring a good metabolic control, beneficial effects on renal (17), ocular (18) and carotid (19) blood flow at CDU have been demonstrated. Retinal microcirculation improvement is particularly relevant because diabetes is still the major cause of blindness in western countries (75). Other imaging techniques, such as MRI and PET, are under investigation to detect the engrafted islets: both techniques require ex-vivo islets to be labeled before PIPIT with SPIO and fluorine 18 fluorodeoxyglucose (FDG). Labeled islets can be visualized at MRI (40) and PET (42) allowing the monitoring of islet engraftment. DCE-MRI has been also used to study liver perfusion changes after PIPIT and their correlation with graft function (41).
In conclusion, allo-PIPIT is a minimally invasive, innovative and repeatable therapeutic option to treat brittle T1DM, reducing the impact of its complications. Auto-PIPIT, in case of extensive pancreatectomy, prevents pancreatogenic diabetes without need of immunosuppression. Interventional radiology and imaging both play a key role in PIPIT. CDU represents an useful tool to obtain hemodynamic information about portal vein before transplant, to provide real time guidance to the interventional procedure reducing puncture attempts, to early diagnose peri-procedural complications, to monitor hepatic and vascular changes and, in our opinion, to predict the clinical outcome in case of steatosis detection.
AcknowledgementsOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
This study was supported by Juvenile Diabetes Research Foundation (JDRF), Telethon Italy, European Association for the Study of Diabetes (EASD), Ministero dell’Istruzione dell’ Università e della Ricerca (MIUR) of Italy, Ministero della Salute of Italy, Dompè SPA and Associazione Italiana per la Ricerca contro il Cancro (AIRC).
FootnoteOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Introduction
- Criteria of analysis
- Pre-PIPIT imaging
- Interventional procedure
- Early post-PIPIT imaging
- Late post-PIPIT imaging
- Discussion
- Acknowledgements
- Footnote
- References
- Dixon S, Tapping CR, Walker JN, et al. The role of interventional radiology and imaging in pancreatic islet cell transplantation. Clin Radiol 2012;67:923-31. [Crossref] [PubMed]
- Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53. [Crossref] [PubMed]
- Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev 1999;15:42-6. [Crossref] [PubMed]
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384-95. [Crossref] [PubMed]
- Sutherland DE, Goetz FC, Najarian JS. Pancreas transplantation. Clin Endocrinol Metab 1982;11:549-78. [Crossref] [PubMed]
- Sutherland DE, Gruessner AC, Gruessner RW. Pancreas transplantation: a review. Transplant Proc 1998;30:1940-3. [Crossref] [PubMed]
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learnt from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001;233:463-501. [Crossref] [PubMed]
- Smets YF, Westendorp RG, van der Pijl JW, et al. Effect of simultaneous pancreas—kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet 1999;353:1915-9. [Crossref] [PubMed]
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplant on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002;25:906-11. [Crossref] [PubMed]
- Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017;13:268-77. [Crossref] [PubMed]
- Wynn JJ, Distant DA, Pirsch JD, et al. Kidney and pancreas transplantation. Am J Transplant 2004;4:72-80. [Crossref] [PubMed]
- Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 2005;5:2037-46. [Crossref] [PubMed]
- Scharp DW, Lacy PE, Santiago JV, et al. Results of our first nine intraportal islet allografts in type 1, insulin dependent diabetic patients. Transplantation 1991;51:76-85. [Crossref] [PubMed]
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230-8. [Crossref] [PubMed]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy, Lachin JM, Genuth S, et al. N Engl J Med 2000;342:381-9. [Crossref] [PubMed]
- Thompson DM, Meloche M, Ao Z, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 2011;91:373-8. [Crossref] [PubMed]
- Fiorina P, Venturini M, Folli F, et al. Natural history of kidney graft survival, hypertrophy, and vascular function in end-stage renal disease type 1 diabetic kidney-transplanted patients: beneficial impact of pancreas and successful islet cotransplantation. Diabetes Care 2005;28:1303-10. [Crossref] [PubMed]
- Venturini M, Fiorina P, Maffi P, et al. Early increase of retinal arterial and venous blood flow velocities at color Doppler imaging in brittle type 1 diabetes after islet transplant alone. Transplantation 2006;81:1274-7. [Crossref] [PubMed]
- Fiorina P, Folli F, Bertuzzi F, et al. Long-term beneficial effect of islet transplantation on diabetic macro-/microangiopathy in type 1 diabetic kidney-transplanted patients. Diabetes Care 2003;26:1129-36. [Crossref] [PubMed]
- Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation 2008;86:1799-802. [Crossref] [PubMed]
- Helling TS. Surgical management of chronic pancreatitis and the role of islet cell autotransplantation. Curr Surg 2003;60:463-9. [Crossref] [PubMed]
- King J, Kazanjian K, Matsumoto J, et al. Distal pancreatectomy: incidence of postoperative diabetes. J Gastrointest Surg 2008;12:1548-53. [Crossref] [PubMed]
- Parsaik AK, Murad MH, Sathananthan A, et al. Metabolic and target organ outcomes after total pancreatectomy: Mayo Clinic experience and meta-analysis of the literature. Clin Endocrinol (Oxf) 2010;73:723-31. [Crossref] [PubMed]
- Balzano G, Maffi P, Nano R, et al. Extending indications for islet autotransplantation in pancreatic surgery. Ann Surg 2013;258:210-8. [Crossref] [PubMed]
- Venturini M, Angeli E, Maffi P, et al. Technique, complications, and therapeutic efficacy of percutaneous transplantation of human pancreatic islet cells in type 1 diabetes: the role of US. Radiology 2005;234:617-24. [Crossref] [PubMed]
- Gaba RC, Garcia-Roca R, Oberholzer J. Pancreatic Islet Cell Transplantation: An Update for Interventional Radiologists. J Vasc Interv Radiol 2012;23:583-94. [Crossref] [PubMed]
- Rajab A. Islet transplantation: alternative sites. Curr Diab Rep 2010;10:332-7. [Crossref] [PubMed]
- Taylor GD, Kirkland T, Lakey J. Bacteremia due to transplantation of contaminated cryopreserved pancreatic islets. Cell Transplant 1994;3:103-6. [Crossref] [PubMed]
- Owen RJ, Ryan EA, O’Kelly K, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology 2003;229:165-70. [Crossref] [PubMed]
- Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat 1990;27:185-95. [Crossref] [PubMed]
- Low G, Hussein N, Owen RJ, et al. Role of imaging in clinical islet transplantation. Radiographics 2010;30:353-66. [Crossref] [PubMed]
- Baidal DA, Froud T, Ferreira JV, et al. The bag method for islet cell infusion. Cell Transplant 2003;12:809-13. [Crossref] [PubMed]
- Funaki B. Islet cell transplantation. Semin Intervent Radiol 2006;23:295-7. [Crossref] [PubMed]
- Villiger P, Ryan EA, Owen R, et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant 2005;5:2992-8. [Crossref] [PubMed]
- Froud T, Yrizarry JM, Alejandro R, et al. Use of D-STAT to prevent bleeding following percutaneous transhepatic intraportal islet transplantation. Cell Transplant 2004;13:55-9. [Crossref] [PubMed]
- Low G, Hussein N, Owen RJ, et al. Role of imaging in clinical islet transplantation. Radiographics 2010;30:353-66. [Crossref] [PubMed]
- Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060-9. [Crossref] [PubMed]
- Malosio ML, Esposito A, Brigatti C, et al. MR imaging monitoring of iron-labeled pancreatic islets in a small series of patients: islet fate in successful, unsuccessful, and autotransplantation. Cell Transplant 2015;24:2285-96. [Crossref] [PubMed]
- Esposito A, Palmisano A, Maffi P, et al. Liver perfusion occurring during pancreatic islet engraftment: a dynamic contrast-enhanced magnetic resonance study. Am J Transplant 2014;14:202-9. [Crossref] [PubMed]
- Li J, Karunananthan J, Pelham B, et al. Imaging pancreatic islet cells by positron emission tomography. World J Radiol 2016;8:764-74. [Crossref] [PubMed]
- Eckhard M, Martin I, Eich T, et al. Incidence of cytomegalovirus infections after immunosuppression induction in clinical islet transplantation and impact on graft function. Transplant Proc 2002;34:1922-4. [Crossref] [PubMed]
- Low G, Hussein N, Owen RJ, et al. Role of imaging in clinical islet transplantation. Radiographics 2010;30:353-66. [Crossref] [PubMed]
- Kilworth L, Crane D, Masters C. The influence of insulin on the flux of lipid metabolism in vivo. Biochem Int 1985;10:539-47. [PubMed]
- Markmann JF, Rosen M, Siegelman ES, et al. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: a functional footprint of islet graft survival? Diabetes 2003;52:1591-4. [Crossref] [PubMed]
- Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes 2004;53:1311-7. [Crossref] [PubMed]
- Maffi P, Angeli E, Bertuzzi F, et al. Minimal focal steatosis of liver after islet transplantation in humans: a long-term study. Cell Transplant 2005;14:727-33. [Crossref] [PubMed]
- Jackson S, Mager DR, Bhargava R, et al. Long-term follow-up of hepatic ultrasound findings in subjects with magnetic resonance imaging defined hepatic steatosis following clinical islet transplantation: a case-control study. Islets 2013;5:16-21. [Crossref] [PubMed]
- Leitão CB, Peixoto EM, Westphalen AC, et al. Liver fat accumulation after islet transplantation and graft survival. Cell Transplant 2014;23:1221-7. [Crossref] [PubMed]
- Ong SL, Pollard C, Rees Y, et al. Ultrasound changes within the liver after total pancreatectomy and intrahepatic islet cell autotransplantation. Transplantation 2008;85:1773-7. [Crossref] [PubMed]
- Venturini M, Maffi P, Querques G, et al. Hepatic steatosis after islet transplantation: Can ultrasound predict the clinical outcome? A longitudinal study in 108 patients. Pharmacol Res 2015;98:52-9. [Crossref] [PubMed]
- Venturini M, Angeli E, Maffi P, et al. Liver focal fatty changes at ultrasound after islet transplantation: an early sign of altered graft function? Diabet Med 2010;27:960-4. [Crossref] [PubMed]
- Rajagopalan S, Harrison DG. Reversing endothelial dysfunction with ACE inhibitors. A new trend. Circulation 1996;94:240-3. [Crossref] [PubMed]
- Petrelli A, Maestroni A, Fadini GP, et al. Improved function of circulating angiogenic cells is evident in type 1 diabetic islet-transplanted patients. Am J Transplant 2010;10:2690-700. [Crossref] [PubMed]
- Venturini M, Losio C, Del Maschio A, et al. Kidney-pancreas transplantation does not improve retinal arterial flow velocities in type 1 diabetic uremic patients. Transplantation 2010;89:261. [Crossref] [PubMed]
- Fiorina P, Folli F, Zerbini G, et al. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol 2003;14:2150-8. [Crossref] [PubMed]
- Fiorina P, Venturini M, Folli F, et al. Natural history of kidney graft survival, hypertrophy, and vascular function in end-stage renal disease type 1 diabetic kidney-transplanted patients: beneficial impact of pancreas and successful islet cotransplantation. Diabetes Care 2005;28:1303-10. [Crossref] [PubMed]
- Fiorina P, Gremizzi C, Maffi P, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care 2005;28:1358-65. [Crossref] [PubMed]
- Korsgren O, Lundgren T, Felldin M, et al. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 2008;51:227-32. [Crossref] [PubMed]
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318-30. [Crossref] [PubMed]
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011;11:364-74. [Crossref] [PubMed]
- Dixon S, Tapping CR, Walker JN, et al. The role of interventional radiology and imaging in pancreatic islet cell transplantation. Clin Radiol 2012;67:923-31. [Crossref] [PubMed]
- Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830-5. [Crossref] [PubMed]
- Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012;12:1576-83. [Crossref] [PubMed]
- Berney T, Ferrari-Lacraz S, Bühler L, et al. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark. Am J Transplant 2009;9:419-23. [Crossref] [PubMed]
- Chang CA, Lawrence MC, Naziruddin B. Current issues in allogeneic islet transplantation. Curr Opin Organ Transplant 2017;22:437-43. [Crossref] [PubMed]
- Shapiro AM, Lakey JR, Rajotte RV, et al. Portal vein thrombosis after transplantation of partially purified pancreatic islets in a combined human liver/islet allograft. Transplantation 1995;59:1060-3. [Crossref] [PubMed]
- Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation 2008;86:1799-802. [Crossref] [PubMed]
- Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 2012;214:409-24. [Crossref] [PubMed]
- Chinnakotla S, Radosevich DM, Dunn TB, et al. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg 2014;218:530-43. [Crossref] [PubMed]
- Garcea G, Weaver J, Phillips J, et al. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consecutive patients. Pancreas 2009;38:1-7. [Crossref] [PubMed]
- Argo JL, Contreras JL, Wesley MM, et al. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg 2008;74:530-6. [PubMed]
- Weimar B, Rauber K, Brendel MD, et al. Percutaneous transhepatic catheterization of the portal vein: A combined CT- and fluoroscopy-guided technique. Cardiovasc Intervent Radiol 1999;22:342-4. [Crossref] [PubMed]
- Goss JA, Soltes G, Goodpastor SE, et al. Pancreatic islet transplantation: the radiographic approach. Transplantation 2003;76:199-203. [Crossref] [PubMed]
- Casey JJ, Lakey JR, Ryan EA. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation 2002;74:913-5. [Crossref] [PubMed]
- Abbate M, Cravedi P, Iliev I, et al. Prevention and treatment of diabetic retinopathy: evidence from clinical trials and perspectives. Curr Diabetes Rev 2011;7:190-200. [Crossref] [PubMed]