Metachronous bilateral ectopic breast carcinoma: a case report
Introduction
Ectopic breast tissue (EBT) is a rare diagnosis, occurring in 0.2–6% of the population. EBT is an expression of incomplete embryological involution of the primordial mammary cellular groups located along the milk lines. The most frequent location of aberrant tissue is in the axilla (60–70% of all cases), followed by the vulva and chest region. In the European population the incidence is estimated to the 0.22%, in the greatest part sporadic; familiar transmission might be seen in 10% of cases (1-4). EBT develops under hormonal impulses, such as mammary gland, and undergoes physiologic changes as a complete functioning breast.
EBT is subject to the pathological alterations of normal breast, including carcinoma, although carcinoma of EBT is uncommon, accounting for 0.2–0.6% of all breast cancers. The most common histotype discovered in the ectopic breast carcinoma is ductal carcinoma (45–80% of all cases), followed by lobular carcinoma that represents less than 10% (5,6). The authors present a case of a 76-year-old female patient, with a rare lobular carcinoma of the abdominal wall EBT, treated with surgery and adjuvant therapy.
Case presentation
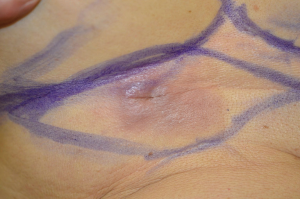
A 76-year-old white woman presented to our Department of Surgery in July 2014 for a palpable, painless mass in abdominal wall, occasionally detected by the patient herself. Her family history was negative for breast cancer and ovarian cancer. The patient had her first menses at the age of 12 years, parity was negative, menopause at the age of 53 years old, in the post-menopausal period she took hormone replacement therapy (HRT) for 23 years. Her medical history was significant for hypertension, atrial tachycardia, chronic gastritis and osteoarthritis. Her bilateral breast and axillary clinical examination was normal. The right ectopic breast mass was located anterior to the costal arch, its surrounding skin showed dark brownish discoloration, as a palpable subcutaneous mass. The local physical examination showed a hyperemic area with lymphangitis, subcutaneous mass hard-fibrous texture of 8 cm × 4 cm, at the level of the right abdominal wall in the epigastric region, located 3 cm caudal to the inframammary fold ipsilateral (Figures 1,2). Diagnostic assessment of the ectopic breast lesion was performed, including breast and loco-regional ultrasound (US), mammography, breast magnetic resonance imaging (MRI). The US showed an 80-mm solid hypoechoic lesion with inhomogeneous echo structure and expansive growth pattern, there was mild hypervascularity appreciated, with smooth well-demarcated margins and small areas of irregularity along the lateral margins; mammographic slides, breast and axillary US were negative for breast mass.

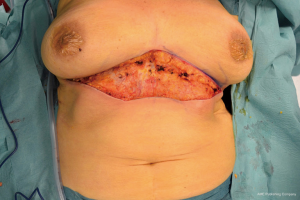
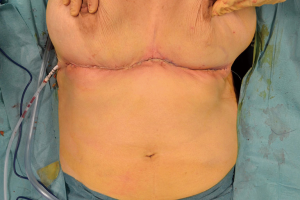
All these clinical and radiologic data were highly suggestive for a malignant lesion arose in the EBT. For a confirmed diagnosis was performed a 16-gauge core needle biopsy of the palpable mass. Histological examination demonstrated a dermo-hypodermic localization of breast lobular carcinoma moderately differentiated; immunohistochemical exam revealed immunohistochemistry revealed estrogen receptor (ER) and progesterone receptor (PR) positive >95%, and human epidermal growth factor receptor 2 (HER2) negative. Abdominal US, chest X-ray and whole-body bone scintigraphy were normal. This case was staged as T4bN0M0 lobular carcinoma of EBT. After a multi-disciplinary evaluation, patient underwent to an extended excision of the tumor with upper abdominoplasty and right axillary sentinel lymph node biopsy was performed using radioactive tracer (Figures 3,4). Sentinel lymph node was mapping a day before surgery with peritumoral radiotracer injection and one right axillary sentinel node was identified with gamma camera during surgery.
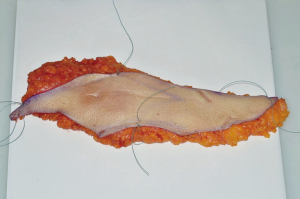
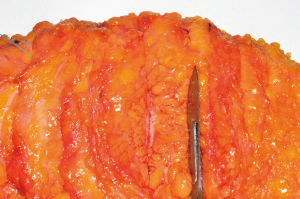
The postoperative course was uneventful. Final histopathological study reported a yellow-withe area of 8 cm × 3 cm, with poorly circumscribed margins, hard to cut with clear surgical margins (Figures 5,6). It was confirmed the previous histological diagnosis of invasive lobular carcinoma G2 infiltrating the dermis and subcutaneous tissue; the only one axillary sentinel lymph node was negative. The complementary immunohistochemistry showed positive for cytokeratin ABI and AE3, negative for E-cadherin, p53 and HER2; Ki67 was 6%, ER 98% and PR 80%.
It was proposed and accepted adjuvant hormonal treatment with letrozole (2.5 mg/day) and adjuvant radiotherapy that the patient refused. Therefore, it was started a close clinical follow-up every 3 months.
In January 2016, 19 months after surgery, it was observed in the left upper quadrant the abdominal wall a 1.5-cm subcutaneous mass with an initial cutaneous infiltration. The lesion was promptly subjected to excisional biopsy under local anesthesia and the histopathology study reported dermo-hypodermic lobular carcinoma infiltration.
Bilateral mammography, breast and axillary US were done, as well as MRI, all reported without breast disease, a total body computed tomography (CT) was negative for metastases.
Once admitted in our clinic and evaluated by the tumor board, the patient underwent an extended excision of the surgical scar, a left axillary sentinel lymph node radioguided biopsy and total axillary lymph node dissection because the sentinel lymph node was positive for macrometastasis at the frozen sections.
The histopathological study revealed no residual tumor in the surgical specimen, 11 out of 15 lymph nodes were metastatic. The new lesion was staged as a pT4bN3M0R0 (ER, 98%; PR, <1%; Ki67, 12%; p53, negative; HER2, negative).
Adjuvant chemotherapy based in four cycles of cyclophosphamide and epirubicin followed by weekly paclitaxel were offered: unfortunately, chemotherapy was suspended for side effects and poor tolerance. Also, radiotherapy at 50 Gray was planned for lymph node positivity but it was refused by the patient. This management plan is in agreement with the literature (5-7). The patient is in follow-up 18 months after.
Discussion
Accessory breast tissue occurs in axilla in about 70% of cases. Another 20% occurs in thoracic or abdominal portions of the milk line, often on the left side of the body just below the inframammary crease. In our case it was located in the bilateral part of abdominal wall in epigastric region. In 1915, Kajava published a classification system for EBT that remains in use today. Class I consists of a complete breast with nipple, areola, and glandular tissue. Class II consists of nipple and glandular tissue but no areola. In class III there is an areola and glandular tissue but no nipple, in class IV glandular tissue only, class V nipple and areola but no glandular tissue (pseudomamma). Class VI consists of a nipple only (polythelia), in class VII there is only an areola (polythelia areolaris), and class VIII shows of a patch of hair only.
Ectopic tissue could be composed of only subcutaneous glandular tissue or from full areola and nipple tissue. The accessory breast structure most frequently encountered is a small nipple, that can be confused with a skin nevus (8,9). The anterior chest wall is a rare location of ectopic breast cancer. In a review article, Da Silva et al. reported 12 cases between 1966 and 2007 (10). To our knowledge, the patient we operated could be classified as a very rare case of bilateral cancer arising in the EBT located in the anterior abdominal wall. Carcinomas in this area are reported to be very rare. Despite the fact that carcinoma that occurs in EBT is rare, all types of breast cancer have been reported in the literature such as ductal, lobular, medullary, mucinous and papillary carcinomas (11-13).
Common differential diagnosis includes lipoma, benign lymphadenopathy, lymphadenitis, metastatic carcinoma, lymphoma, and hydradenitis suppurativa. In the case of a pigmented axillary lesion, the differential diagnosis includes seborrheic keratosis, fibromas, and intradermal nevus (14-15).
The clinical evaluation and staging of EBT cancer should follow the same guidelines as for anatomic breast cancer. In case of axillary EBT, an MRI of the anatomic breast should be made to exclude a primary breast cancer (5,6). For located stage, the best treatment option is surgical excision of primary tumor with sentinel lymph node biopsy +/− axillary dissection, according to the nodal status, followed by radiotherapy, chemotherapy or endocrine therapy. The principles of adjuvant treatment are the same as for anatomic breast cancer and post-operative radiotherapy to the tumor site and regional lymph nodes is indicated. The rarity of the disease and the few available follow-up data do not allow for accurate estimation of prognosis, but EBT seems to have a poorer prognosis than cancer in normal breast gland. The prognosis could be poor because early diagnosis is difficult. What makes our case especially interesting is patient’s prior history of a contralateral hormone receptor-positive ectopic breast cancer. This is a rare case describing a metachronous bilateral ectopic breast cancer. During follow-up, an accurate clinical and instrumental evaluation is important in order to identify an early diagnosis of a second ectopic tumor along the milk line or locoregional recurrence.
Conclusions
EBT is found in up to 0.2–6% of the population and may undergo malignant transformation. The possibility of a second primary ectopic breast cancer also needs to be kept in mind in patients with prior history of ectopic breast cancer when a new malignant lesion is found in the milk line area. It is important to perform a careful follow-up of these patients, especially because the natural history of this rare disease is not well defined yet. Indeed, data regarding survival and local recurrence in patients with ectopic breast cancer could not be obtained with certainty due to its rare incidence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Sadler TW, Leland J. Langman's Medical Embryology. 5th edition. Baltimore, MD: Williams & Wilkins, 1985.
- Burdick AE, Thomas KA, Welsh E, et al. Axillary polymastia. J Am Acad Dermatol 2003;49:1154-6. [Crossref] [PubMed]
- Rho JY, Juhng SK, Yoon KJ. Carcinoma originating from aberrant breast tissue of the right upper anterior chest wall: a case report. J Korean Med Sci 2001;16:519-21. [Crossref] [PubMed]
- Roorda AK, Hansen JP, Rider JA, et al. Ectopic breast cancer: special treatment considerations in the postmenopausal patient. Breast J 2002;8:286-9. [Crossref] [PubMed]
- Bakker J, Sataloff D, Haupt H. Breast cancer presenting in aberrant axillary breast tissue. Commun Oncol 2005;2:117-20. [Crossref]
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol 2011;20:35-42. [Crossref] [PubMed]
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol 1994;3:295-304. [Crossref] [PubMed]
- Kajava Y. The proportions of supernumerary nipples in the Finnish population. Duodecim 1915;31:143-70.
- Urbani CE, Betti R. Accessory mammary tissue associated with congenital and hereditary nephrourinary malformations. Int J Dermatol 1996;35:349-52. [Crossref] [PubMed]
- da Silva BB, dos Santos AR, Pires CG, et al. Ectopic breast cancer in the anterior chest wall: a case report and literature review. Eur J Gynaecol Oncol 2008;29:653-5. [PubMed]
- Shin SJ, Sheikh FS, Allenby PA, et al. Invasive secretory (juvenile) carcinoma arising in ectopic breast tissue of the axilla. Arch Pathol Lab Med 2001;125:1372-4. [PubMed]
- Chung-Park M, Zheng Liu C, Giampoli EJ, et al. Mucinous adenocarcinoma of ectopic breast tissue of the vulva. Arch Pathol Lab Med 2002;126:1216-8. [PubMed]
- Pardo M, Silva F, Jiménez P, et al. Mammary carcinoma ine ectopic breast tissue. A case report. Rev Med Chil 2001;129:663-5. [PubMed]
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol 2007;34 Suppl 1:9-13. [Crossref] [PubMed]
- Avilés Izquierdo JA, Martínez Sánchez D, Suárez Fernandez R, et al. Pigmented axillary nodule: carcinoma of an ectopic axillary breast. Dermatol Surg 2005;31:237-9. [Crossref] [PubMed]