Clinical profile and long-term follow-up of 32 patients with postoperative permanent hypoparathyroidism
Introduction
The prevalence of postoperative permanent hypoparathyroidism (PH) varies from 0–3% according to a recent literature review (1) to 6–12% according to national registries and multicenter studies. This wide variation can be attributed to lack of proper definitions, defective follow-up and conflicts of interest. There is a lack of consensus on PH definition (2). European guidelines define PH as low levels of parathyroid hormone (PTH) and need for replacement therapy 6 months after surgery (3) whereas other reports consider PH 1 year after thyroidectomy (2,4). We did propose to use the 1-year deadline since according to our data (unpublished) about one third of patients recover from protracted hypoparathyroidism after 6 months.
Once PH is diagnosed, it requires lifelong medical control involving a close monitoring of calcium levels—to prevent hyper/hypocalcemic events—and the renal function. According to the European Society of Endocrinology (ESE) Clinical Guideline (3), biochemical monitoring of these patients should include serum calcium (sCa) levels, phosphate, magnesium, creatinine and estimated glomerular filtration rate every 3–6 months, and 24-h urinary calcium excretion once a year or every second year. Renal imaging is recommended in case patient develops symptoms of renal stone disease or if serum creatinine levels start to rise and they advise against routine monitoring of bone mineral density by dual energy X-ray absorptiometry (DXA) scans. The American Association of Clinical Endocrinologist (AACE) and the American College of Endocrinology (ACE) published a Disease State Clinical Review (4) in which the frequency of biochemical surveillance is not specified, but their recommendations regarding periodic renal ultrasound and DXA scans were similar.
There is wide consensus (3-5) that the best replacement treatment for PH consists of calcium salts associated with active vitamin D analogues to maintain sCa concentrations between 2 mmol/L (8 mg/dL) and the lower limit of the reference avoiding hypocalcemic symptoms. No formal recommendations have been agreed regarding the most appropriate vitamin D analogue. Thiazide diuretics can be added in cases with difficult calcium control or with elevated urinary calcium levels.
Recently, substitutive therapy with recombinant human PTH has been reported as an alternative or complement to standard supplementation (6). The REPLACE trial (7) showed that half of patients receiving recombinant human PTH could reduce more than 50% their calcium and vitamin D requirements. Similar data were reported in a cohort study (8). It has been suggested that recombinant PTH may have a positive impact in bone microarchitecture and decrease urinary calcium excretion (6,8). Recombinant PTH has not been implemented, however, as a routine treatment and it is likely that recommendation for its use will be restricted to those patients who cannot be properly controlled under conventional therapy (3).
Limited data suggest that the quality of life may be compromised in patients with PH despite optimization of their biochemical values (9,10). In the PARADOX study (11), 374 American patients affected of PH ≥6 months were interviewed. Of those polled, 60% agreed that PH management was more difficult than expected, 72% experienced more than ten symptoms and 80% required a hospital admission. Only 18% were satisfied with the current treatment and more than one third experienced adverse effects. These data need to be interpreted with caution since they may also reflect poor medical control. In fact, the PARADOX study gives no consistent information on the quality of medical control, compliance, comorbidities or follow-up protocols. A surprising data reported in this study is the low (0.5%) proportion of patients followed up by surgeons.
A large case-control Danish study conducted by Underbjerg et al. (12) showed that PH patients experienced an increased risk of renal stones, renal failure and seizures but no increased risk for cardiac arrhythmias nor cardiovascular disease or death. One year later, the same authors reported that the risk of fractures at the upper extremities was significantly decreased in PH (13). Compared with controls, however, patients had a significantly increased risk of hospitalization due to infections and depression/bipolar affective disorders.
The aim of the present study was to assess the incidence of complications derived from PH status in our closely followed cohort of PH patients, analyze the impact of treatment received and define the influence of these adverse events on the quality of life.
Methods
The study was designed as a cross-sectional review of a single center prospectively maintained clinical database of consecutive patients receiving either first-time total thyroidectomy or thyro-parathyroidectomy between 1998 and 2013. All the procedures were performed by the same team of experienced endocrine surgeons at the Hospital Universitari del Mar in Barcelona, Spain, a tertiary referral center for Endocrine Surgery. Exclusion criteria were Graves’ disease (usually treated with subtotal resection), Dunhill procedure, reoperations and patients treated with calcium for other reasons such as osteoporosis and malabsorption.
Surgical technique
Total thyroidectomy was performed for either multinodular goiter or thyroid cancer, the latter was associated with routine central compartment node dissection and selective modified radical lateral neck dissection when indicated. Parathyroidectomy was associated to total thyroidectomy in the presence of parathyroid adenoma. Parathyroid glands were looked for in their orthotopic position and mobilized using fine forceps and low-intensity electro-dissection using a fine disposable electrosurgical electrode needle (UTAH OptimicroTM, Utah Medical Products, Midvale, Utah, USA). In all cases, identification of parathyroid glands was based solely on visual macroscopic features. Ligation of the inferior thyroid artery was always carried out at the level of the distal branches, close to the thyroid gland. Parathyroid glands that could not be preserved in situ were autotransplanted. The glands were chopped into 1 mm3 fragments and buried into several pockets in the ipsilateral sternocleidomastoid muscle.
Patient management and follow-up
According to our policy, all patients presenting postoperative hypocalcemia are followed in our clinic until resolution or PH is diagnosed. We then follow patients with PH every 6 months monitoring intact PTH (iPTH), sCa, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and kidney function (creatinine and estimated glomerular filtration). Typically, patients with PH receive treatment with calcium salts (1.5–3.0 g of calcium/day) and vitamin D analogues: calcitriol (Rocaltrol®), 0.25–1.00 µg/day and/or calcifediol (Hidroferol®), 266 mg 1 to 3 times/week. There was no formal criterion to use one or the other.
Some patients agreed to undergo additional testing with kidney ultrasound, bone mineral density scan (DXA) and 24-h urine calcium test. Perceived quality of life was assessed in 19 patients using an adaptation of the Spanish version of SF-36 Health (14). Data was collected by a trained interviewer who read orally each of the questions and the possible answers letting the patients select the most correct answer in each case.
Episodes (events per patient and density of events) of hypocalcemia and hypercalcemia were recorded. Long-term complications such as basal ganglia calcifications, nephrolithiasis and nephrocalcinosis, fractures and osteoporosis were studied were also recorded. Current medication at the time of the last follow-up visit was recorded to compare the prevalence of complications of treatment with the two different vitamin D analogues (calcitriol vs. calcifediol). iPTH levels were determined using either an immune-radiometric 2nd generation assay using IRMA Total Intact assay (Scantibodies Laboratory Inc. Santee, CA, USA) or a solid-phase, two-site chemiluminescent enzyme-labeled assay IMMULITE 2000 Intact PTH assay (Siemens Healthcare Diagnostics Spain, Madrid, Spain); with a normal range of 13–65 pg/mL and a detection limit of 3 pg/mL.
Definitions
PH was defined as a subnormal iPTH concentration (<13 pg/mL) and need for calcium replacement with or without calcitriol for more than 1 year after surgery. Hypocalcemia was defined as a sCa <8 mg/dL (2 mmol/L) and/or the presence of acral paresthesias and muscle spasms and hypercalcemia as sCa >10.5 mg/dL. Renal failure was considered when the last creatinine value was >1.2 mg/dL and/or an estimated glomerular filtration rate <60 mL/min/1.73 m2. SF-36 questionnaire results were weighed by age and sex in each dimension and compared with normal reference data from the Spanish population (15).
Statistical analysis
A database created with FileMaker Pro 8 software (Santa Clara, CA, USA) was prospectively maintained. Demographic, clinical, surgical, histopathology and biochemical variables relevant for the diagnosis and management of postoperative hypocalcemia were routinely recorded. Clinical events during follow-up are expressed as events/1,000 patient-days. Statistical analyses were performed using SPSS IBM Statistics v.22.0 (Armonk, NY, USA). The normal distribution of quantitative variables was assessed with the Kolmogorov-Smirnov test. Comparison of proportions was investigated with chi-square or exact Fisher tests as appropriate. For quantitative variables, the unpaired Student’s t-test was used. Values are expressed as mean ± standard deviation (SD). Statistical significance was set at P<0.05.
Results
The study cohort included 32 patients: 3 men and 29 women with a mean age of 51.2±15.2 years. One patient died during follow-up for causes unrelated to PH and was not excluded. Thyroidectomy was performed for benign goiter in 22 (68.8%) patients and for thyroid cancer in 10 (31.3%) patients. Three or four parathyroid glands were preserved in situ in 81% of patients. Parathyroid transplantation had been carried out in 13 (40.6%) patients. The mean length of follow-up was 78±68 months (range, 12–168 months) for a total of 70,080 days PH patient/days (Table 1).
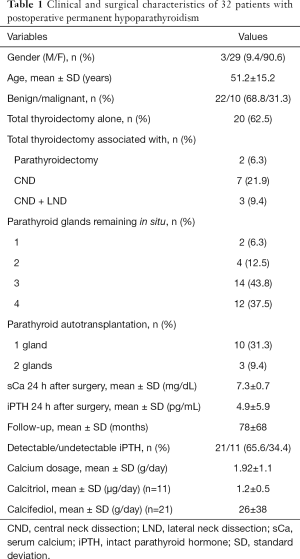
Full table
Adverse clinical events
A total of 48 clinical adverse events were diagnosed in 18 (56.3%) patients (Table 2). The most frequent events requiring medical attention were renal failure (15.6%) and clinical hypocalcemia (15.6%). The most prevalent consultation in the Emergency Department was for clinical hypocalcemia.
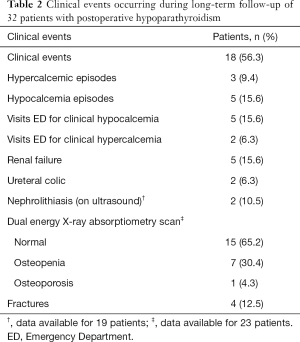
Full table
Complications analyzed according to the type of vitamin D analogue
At the last follow-up visit, patients treated with calcitriol showed a higher serum creatinine concentration than those receiving calcifediol. Of the 32 patients, only one was receiving calcium supplements alone. Although the difference was not statistically significant, renal failure (creatinine >1.2 mg/dL) at the time of the last follow-up visit was twice as common in the calcitriol (27%) than in the calcifediol group (10%). Patients treated with calcitriol showed poorer control of sCa concentrations and had more calcium measurements outside the normal range than those treated with calcifediol. sCa minimum values were noticeably worse in patients treated with calcitriol (Table 3). Clinical hypocalcemic events were almost 5 times more common in the calcitriol group but no differences were observed in the number of visits for clinical hypercalcemia (Table 3). Calcitriol treated patients presented a density of 2.87 episodes of hypocalcemia per 10,000 PH patient/days and four patients of this group sought medical assistance due to overt clinical symptomatology. On the other hand, patients who received calcifediol presented a density of 2.15 episodes of hypocalcemia per 10,000 days and 0.48 episodes of hypercalcemia per 10,000 days and only one patient required medical assistance (Table 3).
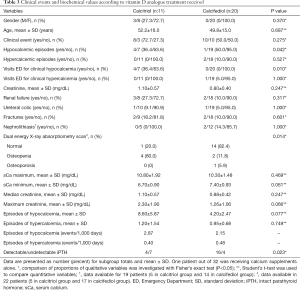
Full table
More intensive treatment with calcium was prescribed for patients with undetectable iPTH than for patients with low but detectable iPTH (2.6±0.9 vs. 1.6±1.0 g/day, respectively; P=0.013). Similarly, vitamin D analogues dose was higher in the group with undetectable iPTH than in the detectable iPTH group: 1.59±0.8 vs. 0.11±0.2 µg/day of calcitriol, P=0.076 and 42.5±49.4 vs. 17.8±28.6 mg/day of calcifediol, P=0.170.
Assessment of quality of life
There was a consistent non-significant decrease of the punctuation for the perceived quality of life in patients with PH for most of the dimensions (6 out of 8) compared with the mean for the Spanish population. The only dimension reaching significance was the emotional role (Table 4).
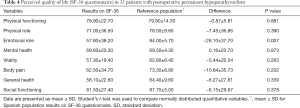
Full table
Discussion
This study on postoperative PH is one the few reporting close monitoring of long-term PH. The follow-up carried out at our unit differs from previous publications due to the strict and careful clinical control and search for differences in the long-term complications in relation to the medical treatment received and the impact of this condition on quality of life. It gives new insights on the consequences of prescribing calcitriol or calcifediol, two of the currently available active vitamin D analogues.
Unlike previous reports (11,16,17), our analysis shows the absence of significant differences in the perceived quality of life of patients with PH compared to the average Spanish population for all the dimensions except for emotional role. Patients in this cohort often reported that emotional problems interfered significantly with daily life the activities or their work, including the reduction of time spent on these activities. This often resulted in a lower performance than expected and decreased job dedication. In contrast, in a multicenter case-control study (18) of 340 patients with postsurgical PH, 47% of patients referred that their health was much worse than before surgery reporting a significantly lower mean score compared to the control group for the eight dimensions. Astor et al. (17) studied a cohort of 283 Norwegian patients and reported that patients with postsurgical hypoparathyroidism scored worse than those with nonsurgical hypoparathyroidism and pseudo- hypoparathyroidism, especially on physical health. The difference might be explained on the basis of a more intense and personalized monitoring of patients in our unit ensuring better treatment control which differs from results obtained from patients retrieved from multicenter registries (13,17,19).
Previous studies have reported a tendency of PH patients to develop renal failure. This may be linked to treatment prescription as calcium and vitamin D analogues increase the risk of hypercalcemia and hypercalciuria eventually leading to nephrocalcinosis or nephrolithiasis.
Mitchell et al. (19) found that 41% of patients had an estimated glomerular filtrate consistent with chronic kidney disease stage 3 or higher and isolated age, duration of disease, estimated proportion of time with sCa higher than 9.5 mg/dL as predictors for impairment of renal function. In a cohort study of the National Institutes of Health, 33% of cases presented decreased glomerular filtration rate (20). The prevalence of renal failure in the present study was 15.6%; 2-fold in patients treated with calcitriol in comparison with those treated with calcifediol. This may be due to calcitriol mechanism of action, as it is the most active and fast-acting analogue of vitamin D.
The present study demonstrates fluctuations of sCa concentrations over time including episodes of hypocalcemia and hypercalcemia occasionally linked to clinical manifestations requiring hospital admission. These were more prevalent in the group of patients treated with calcitriol. These adverse events could also be attributed to the mechanism of action of vitamin D analogues. Therefore, the noticeable proportion of patients with imbalanced sCa levels and renal failure suggests that monitoring and optimizing therapy is of critical importance for patients with PH in order to preserve the renal function. This may be even more relevant in children (21).
Nephrolithiasis and renal colic are well-known complications of hypoparathyroidism due to an increase calcium-phosphate product. Loss of renal PTH action decreases renal tubular reabsorption and excretion of phosphate causing hypercalciuria and hyperphosphatemia (9).
The prevalence in our cohort was similar to that of 10% reported in one cross-sectional study of 25 patients (20). In another cohort study, from 54 patients who had renal imaging at least once, 31% presented either nephrolithiasis or nephrocalcinosis (19).
Long-term hypoparathyroidism leads to a profound reduction in bone remodeling. Subjects with PH typically have bone mineral densities above average at all skeletal sites, with greatest scores observed at the lumbar spine. Volumetric bone mineral density at cancellous and cortical compartments, as well as cortical area and thickness, are higher in PH subjects than in controls (22). Therefore, bone fractures and osteoporosis are diagnosed in only a few patients with this condition. In fact, the opposite may be true. A study of historic cohorts showed that patients with PH seemed to be protected against upper extremity fractures (13). Interestingly, low mineral density was more common in patients on calcitriol which is probably the vitamin D analogue with higher bone resorption activity.
A limitation of this study was the relatively small sample of patients recruited, since in our Endocrine Surgery unit rates of PH have been consistently kept around 4% of total thyroidectomies over time. Actually, PH was designed as an orphan disease by the European Commission in January 2014 (EU/3/13/1210). Patients in this cohort, however, were followed according to an established protocol that ensures an intense and personalized monitoring, resulting in a near to optimal treatment control. Thus, they appear to be less severely affected by PH and with lower rates of complications than patients reported in large multicenter studies (13). A second limitation is that data gathering was conditioned by the willingness of participants to be interviewed and tested (bone densitometry, renal ultrasound). This implies that, for some results, statistical significance was not achieved despite potentially relevant differences found between treatment groups. A second interview round is being planned to obtain the missing data.
In summary, this study records the impact that long-term PH has on clinical events, metabolic parameters and on the perceived quality of life, in patients receiving a close monitorization under current standard treatment. The low proportion of patients with renal failure and presenting episodes of sCa concentrations above or below the reference values suggests that monitoring and optimizing medical therapy is of critical importance to avoid long-term complications. We also provide data showing a higher rate of long-term adverse events in patients treated with calcitriol in comparison with calcifediol. Finally, the study emphasizes the need for longitudinal studies and further research to quantify the impact of the disease on patient’s quality of life and the development of new effective strategies in the prevention of long-term complications.
Acknowledgements
We would like to thank Jordi Alonso Caballero and Gemma Vilagut Saiz from Epidemiology and Public Health Department, IMIM (Hospital del Mar Medical Research Institute) for their kind advise in the assessment of SF-36 questionnaires.
Footnote
Conflicts of Interest: A Sitges-Serra is currently consulting for SHIRE. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the Hospital del Mar and was conducted in accordance with the Declaration of Helsinki of 1964 (revised 2008). All participants provided informed consent before participation.
References
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015;4:82-90. [PubMed]
- Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology clinical guideline: Treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol 2015;173:G1-20. [Crossref] [PubMed]
- Stack BC, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: Postoperative Hypoparathyroidism - Definitions and Management. Endocr Pract 2015;21:674-85. [Crossref] [PubMed]
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: Summary statement and guidelines. J Clin Endocrinol Metab 2016;101:2273-83. [Crossref] [PubMed]
- Cusano NE, Rubin MR, McMahon DJ, et al. PTH(1-84) is associated with improved quality of life in hypoparathyroidism through 5 years of therapy. J Clin Endocrinol Metab 2014;99:3694-9. [Crossref] [PubMed]
- Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol 2013;1:275-83. [Crossref] [PubMed]
- Rubin MR, Cusano NE, Fan WW, et al. Therapy of hypoparathyroidism with PTH(1-84): A prospective six year investigation of efficacy and safety. J Clin Endocrinol Metab 2016;101:2742-50. [Crossref] [PubMed]
- Hypoparathyroidism Shoback D. N Engl J Med 2008;359:391-403. [Crossref] [PubMed]
- Bilezikian JP, Khan A, Potts JT, et al. Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 2011;26:2317-37. [Crossref] [PubMed]
- Hadker N, Egan J, Sanders J, et al. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the Paradox Study. Endocr Pract 2014;20:671-9. [Crossref] [PubMed]
- Underbjerg L, Sikjaer T, Mosekilde L, et al. Cardiovascular and renal complications to postsurgical hypoparathyroidism: A Danish nationwide controlled historic follow-up study. J Bone Miner Res 2013;28:2277-85. [Crossref] [PubMed]
- Underbjerg L, Sikjaer T, Mosekilde L, et al. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res 2014;29:2504-10. [Crossref] [PubMed]
- Alonso J, Prieto L, Antó JM. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): an instrument for measuring clinical results. Med Clin (Barc) 1995;104:771-6. [PubMed]
- Alonso J, Regidor E, Barrio G, et al. Population reference values of the Spanish version of the Health Questionnaire SF-36. Med Clin (Barc) 1998;111:410-6. [PubMed]
- Cho NL, Moalem J, Chen L, et al. Surgeons and patients disagree on the potential consequences from hypoparathyroidism. Endocr Pract 2014;20:427-46. [Crossref] [PubMed]
- Astor MC, Løvas K, Debowska A, et al. Epidemiology and health-related quality of life in hypoparathyroidism in Norway. J Clin Endocrinol Metab 2016;101:3045-53. [Crossref] [PubMed]
- Winer KK, Ko CW, Reynolds JC, et al. Long-term treatment of hypoparathyroidism: A randomized controlled study comparing parathyroid hormone-(1-34) versus calcitriol and calcium. J Clin Endocrinol Metab 2003;88:4214-20. [Crossref] [PubMed]
- Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507-14. [Crossref] [PubMed]
- Arlt W, Fremerey C, Callies F, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol 2002;146:215-22. [Crossref] [PubMed]
- Levy I, Licht C, Daneman A, et al. The impact of hypoparathyroidism treatment on the kidney in children: Long-term retrospective follow-up study. J Clin Endocrinol Metab 2015;100:4106-13. [Crossref] [PubMed]
- Silva BC, Rubin MR, Cusano NE, et al. Bone imaging in hypoparathyroidism. Osteoporos Int 2017;28:463-71. [Crossref] [PubMed]


