Evidence based outcomes of the American Society of Breast Surgeons Nipple Sparing Mastectomy Registry
Introduction
The American Society of Breast Surgeons (ASBrS) Nipple Sparing Mastectomy Registry (NSMR) is an ongoing, prospective, IRB approved, non-randomized, multi-institutional registry housed within the mastery of surgery, ASBrS. The purpose of the ASBrS NSMR is to prospectively collect data informative of the procedure itself including metrics utilized, techniques utilized, aesthetic outcome and oncologic outcome to provide evidenced based medicine on outcome measures and metrics utilized for the nipple sparing mastectomy (NSM). An NSM by definition entails excision of all breast tissue, including all breast tissue behind the nipple areola complex (NAC), while preserving the overlying skin envelope, including the NAC.
Basic science research has better defined breast anatomy, including the relationship of terminal ductal lobular units to the nipple (1) and nipple microvasculature to ductal anatomy (2). This enhanced understanding of the NAC in combination with outcome data on recurrence (3-8), patient satisfaction and quality of life (9-14), infection complication rates (15-26), reconstruction techniques (24-45), incision recommendations (46-52) with concomitant evolution of indication (15-19,52-54) and oncologic criteria from tumor size and location (55-65) to considering the nipple as just another margin (66) has resulted in a larger population women eligible for NSMs.
Ease and feasibility of this registry was demonstrated in a pilot conducted at Stamford Hospital, CT; Georgetown, Washington DC; and Columbia University College of Physicians & Surgeons, NY. The registry is ongoing for 10 years with a target N of 2,000 participating patients. Participating surgeons routinely offer NSM, have performed at least 3 prior to registry participation, are enrolled in the Mastery of Breast Surgery Program, and in accordance with the following: IRB approval, ASBrS NSMR Protocol (v.3 07.2014), Consent to act as a participant in the ASBrS NSMR, and Investigator Agreement. This assessment of data occurred at 74 months initiation of the registry.
Objectives and endpoints
Primary outcome measures include local regional recurrence, disease free survival, and overall survival. Secondary outcome measures include metrics utilized for successful patient selection; patient characteristics; surgical techniques; and aesthetic outcome.
Justification of the registry
The ASBrS is an organization dedicated to furthering knowledge of breast cancer identification, prevention, and treatment. The purpose of this Registry is to provide a large, prospective, non-randomized database of patient characteristics, tumor characteristics, surgical technique, and outcome (both aesthetic and oncologic) of the NSM. This data will contribute to direct comparisons of other surgical procedures for treatment and risk reduction of breast cancer.
Contributors to the ASBrS NSMR include 98 surgeons from 70 sites.
Methods
Data is entered into the ASBrS NSMR, housed within the Mastery of Surgery Program, after patients consent to participation. Each investigator has obtained IRB approval and completed forms of agreement to participate in the ASBrS NSMR. BioStat Inc performed statistics and analysis of data. An internal systematic review defined a subset population (a “per protocol” population) with complete surgical and outcome data, N=1,935 which was used for this analysis. The identified subset was compared to the entire population of entries and found to be consistent thus eliminating concern for bias. Statistical analysis performed via SAS, includes: Fisher’s exact test, pair wise P-value, Bowkers test of Symmetry, and Descriptive analyses.
Results
Patient population
This data set represents a total of 1,935 NSMs performed on 1,170 patients by 98 investigators from 70 institutions/sites over a time period of 6 years and 2 months. Of the 1,935 NSMs: 833 were performed for an indication of cancer [594 invasive carcinoma and 239 ductal carcinoma in situ (DCIS)] and 1,102 were prophylactic. Of the 1,170 total patients, 352 underwent a unilateral and 818 underwent a bilateral NSM. Tumor size ranged from 1 mm to 10 cm, tumor location ranged from <0.5 to >10 cm from the NAC.
Medical comorbidities include hypertension (10.6%), coronary artery disease (0.7%), obesity (6.4%), diabetes mellitus (3%), and chronic obstructive pulmonary disease (COPD) (1%).
The majority of patients, 77%, identified as non-smokers, 5% referred they were current smokers, 4% reported quitting smoking 1–12 months ago, 1.4% reported quitting 1–3 years ago, and 12.6% quit >3 years ago.
NSMs were performed on breasts with varying degrees of ptosis including: grade 1: 33.4%, grade 2: 15.1%, grade 3: 3.9%, pseudo ptosis: 3.5%, and none. Cup size was utilized to reflect the size of the breast. Cup sizes of pre-operative NSMs included: A cup 13.1%, B cup 37.4%, C cup 35.9%, and D+ cup 13.6% (Table 1).
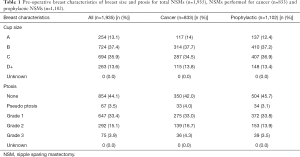
Full table
Pre-operative chemotherapy
A history of pre-operative chemotherapy was reported for 19% (n=367) of NSMs performed: 12.1% (n=234) within 4 months of surgery, 1.1% (n=21) within 4–12 months of surgery, 4.4% (n=85) >1 year prior to surgery, and 1.4% (n=27) with an unknown timeframe. Incidence of Flap infection rate between those with a history of chemotherapy (5.4%) and those without a history of chemotherapy (4.1%) was not statistically significant (P=0.0605).
Prior radiation therapy (XRT)
A minority of NSMs were performed in the setting of a history of XRT; breast (whole breast, partial breast) XRT was 4.2% (n=69), and mantle/other XRT 4.9% (n=89).
Prior XRT and NAC complications
Although the majority of NSMs with a history of XRT either had no NAC complications (85%) or epidermolysis with full recover (96%), there was more NAC necrosis and ischemia requiring surgery than in patients without a history of breast XRT (P=0.0002), mantle/other XRT (P=0.0177).
Prior XRT and flap infection
No significant difference in incidence of flap infection was noted between NSMs with a history of breast XRT (P=0.1657) or mantle/other XRT (P=0.7989).
Prior XRT and cosmesis and patient satisfaction
A history of mantle/other XRT was noted to negatively affect cosmesis (P<0.0001) and patient satisfaction (P=0.0003). Conversely, no effect was noted on NSMs with a history of breast XRT on cosmesis (P=0.2876) or patient satisfaction (P=0.3930).
Oncologic outcome
A 1.4% (n=12) recurrence rate (mean follow-up of 31 months, median follow-up of 27 months), with a range of 9.7–58.3 months since surgery was noted. Of the 12 recurrences: none were at the nipple/NAC, 1 local tumor site, 3 local/not at tumor site, and 8 distant. Recurrences were too few to assign any meaning to tumor biology or characteristics.
An occurrence rate of 0.3% (n=3), 1 local/not at the NAC and 2 distant, at a (mean f/u of 24.3 months, median f/u of 23.3 months) with a range of 21–28.5 months since surgery was noted.
Retro-areola (RA) margin pathology
A separate RA margin specimen was sent for pathology assessment on 96% of NSMs. An RA frozen and permanent pathology assessment was performed on 49% of NSMs; permanent pathology alone for 47% of RA margins, and 4% of NSMs did not have an RA path specimen assessed.
Comparison of frozen section and permanent pathology assessment
Of the 912 RA margin specimens sent only for permanent assessment: 0.9% (n=8) demonstrated invasive cancer, 0.8% (n=7) DCIS, and 0.2% (n=2) atypia, thus there was a 1.9% risk of additional excision (nipple and/or NAC) postoperatively secondary to a permanent path result of invasive cancer or DCIS.
Of the 19 RA margin specimens demonstrating cancer on frozen section: 2 were no evidence of disease (NED) on permanent; 8 were DCIS on permanent; and 9 were cancer on permanent. This correlates to a 10.5% risk of excising the nipple/NAC unnecessarily if acting on frozen section results of cancer. One RA margin frozen assessment of indeterminate demonstrated DCIS on permanent.
NED on RA margin
The majority, 96.6% (n=1,793) of assessed RA margins demonstrated NED on permanent pathology assessment. An additional 9 (0.5%) RAs demonstrated NED on frozen assessment and did not undergo permanent assessment.
Pathology results effecting NAC status
One point two percent of nipples (or NACs) were excised secondary to path results. Intraoperative excision of 0.4% (n=7) nipples and 0.1% (n=2) NACs was performed secondary to preliminary pathology (frozen section) results. Post-operative excision of 0.7% (n=14) NACs and zero nipples was performed secondary to final (permanent) pathology results.
Patient satisfaction and cosmesis
Patient reported satisfaction and surgeon reported cosmesis was rated as excellent, good, fair, or poor. An overall 95% patient satisfaction of excellent/good was reported; excellent (51.2%), good (43.7%), fair (5.1%), and poor (0%). An overall 96% cosmesis of excellent/good was reported; excellent (55%), good (41.4%), fair (3.6%), and poor (0%).
Any variation between patient rated satisfaction and surgeon rated cosmesis?
Both patients and surgeons rated 94% “good/excellent”. The patient rated less than the surgeon 10.9% of the time and the patient rated higher than the surgeon physician 6.5% of the time (P<0.0001, Bowker’s test of Symmetry).
Technique
Incisions
A variety of incisions were utilized including: inframammary (IM) (n=923), radial (n=519), radial with peri-areola extension (n=330), peri-areola ellipse or hemi-batwing (n=26), previous mastopexy scar (n=22), previous lumpectomy scar (n=19), and other (n=96). Radial incision group includes lateral (right breast 9:00, left breast 3:00).
Incision and incidence of flap infection
Incidence of flap infection varied per incision: IM incision (2.4%), radial incision (7.1%), radial with peri-areola extension (4.2%), and other (12.2%). A direct comparison of the three most common incisions: IM incision, radial incision, and radial with peri-areola extension yielded a lower flap infection rate with IM incision, (P<0.0001). A pair wise P=yielded: IM vs. radial incision (P<0.0001); IM vs. radial w/peri-areola extension (P=0.0038); and radial vs. radial w/peri-areola extension (P=0.0027).
Incision and incidence of NAC epidermolysis w/full recovery
Epidermolysis with full recovery varied per incision type: IMF (11.7%), radial w/peri-areola extension (10%), previous mastopexy scar (9.1%), previous lumpectomy scar (10.5%), other (8.3%), peri-areola ellipse or hemi-batwing (7.7%), and radial (7.3%).
Incision and patient satisfaction
In assessing patient satisfaction and incision, the majority of scores were excellent/good of ≥95% including: IM, peri-areola ellipse or hemi-batwing, previous mastopexy scar, and radial. Ninety-two percent excellent/good with a radial incision and 89% excellent/good with a previous lumpectomy scar incision.
Incision and cosmesis
Assessment of cosmesis and incision was consistently >90% excellent/good: IM, peri-areola or hemi-batwing, previous lumpectomy scar, and radial ≥95%; radial with peri-areola extension 94%; and previous mastopexy scar 91%.
Incision utilized for sentinel lymph node biopsy (SLNBx) and axillary lymph node dissection (AXLND)
Both SLNBx’s and AXLND’s were performed via axillary incisions as well as the NSM incision. A SLNBx performed via an axillary incision was associated with a lower incidence of flap infection (3.1%) than if performed via the NSM incision (6.4%) (P=0.0002). No statistically significant difference was noted between AXLNDs performed via an axillary incision (7%) vs. the NSM incision (9.8%) (P=0.2399).
Dissection techniques
Flap dissection technique
NSMs were performed via multiple flap dissection techniques including: electrocautery 43.8%, plasma knife 31%, sharp dissection 11.9%, sharp dissection with tumescent injection 11.7%, and other/unknown 1.6%.
Flap dissection technique and incidence of flap infection
Plasma knife flap dissection demonstrated a lower overall incidence of flap infection (P<0.0001) (Table 2).

Full table
Retro-areola dissection technique
RA dissection performed with a plasma knife was associated with the lowest incidence of flap infection (P<0.0001) (Table 3).

Full table
Infection
NAC ischemia
The majority of NSMs, 86% (n=1,655) had no NAC ischemia (including epidermolysis). An additional 10% (n=193) of NACs experienced epidermolysis with full recovery. A total of 4.5% of NACs had complications (categorized as necrosis or ischemia/epidermolysis requiring surgery): NAC necrosis not requiring surgery 2.9% of NSMs and NAC ischemia requiring surgery 1.6% of NSMs.
NAC post-operative treatments
The NAC of 9.6% of all NSMs received treatment including: topical treatments 4.5%, debridement 2%, NAC excision 2.2%, and other/unknown treatment 0.9%.
Relationship of NAC complication and flap infection
NSMs with a NAC complication (necrosis or ischemia requiring surgery) were noted to have a higher incidence of flap infection (23.0%) than those NSMs without a NAC complication (3.5%) (P<0.0001).
Flap infection
An overall 4.4% rate of flap infection was noted: 0.5% required intravenous (IV) antibiotic (abx) and washout/debridement; 1.8% required abx and implant/tissue expander (TE) removal; 1.4% received oral abx and 0.5% IV abx alone. The majority of NSMs, 95.6%, did not experience a flap infection.
Cosmesis and patient satisfaction in setting of flap infection
Cosmesis and patient satisfaction were rated as 98% and 96% excellent/good respectively in the setting of no flap infection and 78% and 77% respectively with presence of flap infection.
Comparison of non-autologous reconstruction [TE and direct to implant (DI)] incidence of flap infection
The majority of NSMs performed with TE and DI reconstruction did not experience a flap infection (94.6% and 98.5% respectively). A variation in flap infection rate was noted when comparing the non-autologous techniques, TE 5.4% and DI 1.5%, (P<0.0001). Implant removal associated with flap infection was reported as 2.6% for TEs vs. 0.3% of DI (P<0.0001).
Reconstruction
Reconstruction methods utilized
A variety of reconstruction methods were utilized including: TE 63.5% (n=1,229), DI 30.8% (n=596), autologous flap 5.3%, latissimus dorsi (LTD) 0.5% (n=10), transverse rectus abdominis (TRAM) 0.4% (n=7), deep inferior epigastric perforators (DIEP) 4.4% (n=85), other 0.2% (n=4), and unknown 0.2% (n=4). Incision utilized as well as pre-operative cup size or degree of ptosis did not vary per reconstruction type. Patient satisfaction and surgeon rated cosmesis were consistently >90% for each reconstruction type with the exception of TRAM flap (secondary to small n, inappropriate to draw any conclusion regarding TRAM and patient satisfaction/cosmesis).
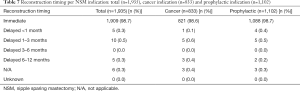
Immediate vs. delayed reconstruction
The majority, 98.7% (1,909 of 1,935), of all NSMs underwent immediate reconstruction. Delayed reconstruction was performed: <1 month: 0.3% (n=5), 1–3 months 0.5% (n=10), 6–12 months, 0.3% (n=5), not applicable (N/A) or unknown 0.3% (n=6).
Flap infection and reconstruction technique
Incidence rate of flap infection per reconstruction type included: DIEP flap 10.6% (9 of 85), LTD flap 10% (1 of 10), TRAM 0% (0 of 7); Other/unknown reconstruction: 0% (0 of 8); DI 1.5% (9 of 587); and TE 5.4% (66 of 1,229) (Table 4).
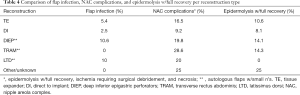
Full table
Non-autologous reconstruction (TE and DI)
A direct comparison of TE and DI yielded a lower incidence of flap infection with DI (2.5%) than TE (5.4%) (P<0.0001). Fewer NAC complications (including epidermolysis with full recovery, necrosis, and epidermolysis/ischemia requiring surgical debridement) were also noted in DI vs. TE (P<0.0001) (Table 5).
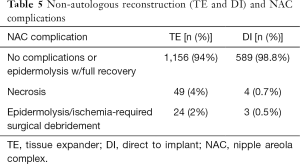
Full table
Comparison of NSMs by indication: prophylactic vs. cancer
No clinically significant variation in breast characteristics i.e., size and ptosis (Table 1), technique i.e., incision utilized or reconstruction type (Table 6) or timing (Table 7), smoking status, or post op nipple sensation was noted between those NSMs performed for indications of cancer or prophylaxis.
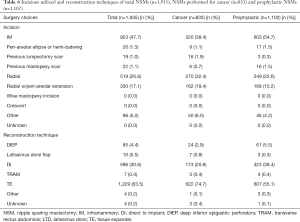
Full table
Full table
Comparison of flap infection incidence between indications
An incidence rate of flap infection of 4.4% (n=85) of the total number of 1,935 was noted. Of the NSMs with a preoperative diagnosis of cancer (invasive carcinoma or DCIS) a 5.2% (43 of 833 NSMs) rate of flap infection was noted. An incidence rate of 4.6% (11 of 239 NSMs) was noted for a preoperative diagnosis of DCIS, and 5.4% (32 of 594 NSMs) was noted for invasive carcinoma. A prophylactic indication was associated with a 3.8% (42 of 1,102) incidence rate of flap infection. A prophylactic indication was associated with a lower incidence of flap infection (3.8%) in comparison to a cancer indication (5.2%) (P=0.0139).
Comparison of cosmesis and patient satisfaction between indications
Cosmesis was rated as excellent/good for 96% of the total population, 95% of NSMs performed for cancer, and 98% of prophylactic NSMs. Patient satisfaction was rated as excellent/good for 95% of total population, 94% of NSMs performed for cancer, and 96% of prophylactic NSMs.
NAC sensation
Post-operative nipple sensation was reported as: full nipple sensation 3.5% of NSMs, partial sensation 20%, no sensation 50%, 2% were excised, and 24% of NSMs were not assessed for sensation. No clinically significant variation was noted between NSMs performed as prophylactic (3.8% full sensation, 18% partial sensation) or cancer (3.1% full sensation, partial sensation 22%). Timing of nipple sensation evaluation was noted as ‘post op’ without a specific time frame from surgery.
Conclusions
NSMs were performed for indications of cancer and prophylaxis utilizing multiple incisions, dissection techniques, and reconstruction options on a variety of breast shapes and sizes with a recurrence rate of 1.4% and an occurrence rate of 0.3% (none at the nipple/NAC), overall patient satisfaction of 95%, cosmesis 96%, 4.4% flap infection rate, 10% epidermolysis with full recovery, and 4.5% NAC complication rate (not including epidermolysis with full recovery).
The data presented here represents the work of many breast and plastic surgeons in private practice, community hospitals, and tertiary care centers across the U.S. demonstrating the feasibility of performing a NSM successfully with high patient satisfaction rates and acceptable infection rates via multiple techniques of dissection, incision placement and reconstruction type. Follow-up will continue to document recurrence and occurrence rates.
Definitions
Ptosis
Ptosis is defined as none, grade 1, 2 or 3, or pseudoptosis. Grade 1 ptosis is defined as the nipple at the level of the IM fold with parenchyma below it; in grade 2 ptosis the nipple is below the level of the IM fold and above the most dependent aspect of the parenchyma; and in grade 3 ptosis the nipple lies below the level of the IM fold and the lowest aspect of breast parenchyma. In a breast with pseudoptosis, the nipple lies at or above the IM fold and the majority of the breast parenchyma is below the fold.
NSM
An NSM entails excision of all breast tissue and preservation of the overlying NAC. When performing a NSM, the dissection plane immediately behind the NAC is immediately adjacent to the dermis. The tissue immediately under the NAC is typically referred to as the RA margin, and is typically separately assessed as a pathology specimen. Excising this margin includes all underlying parenchyma and fat, leaving exposed dermis.
Acknowledgements
(I) Co-investigators of the ASBrS NSMR: Drs: Miral Amin, Preya Ananthakrishnan, Beth Anglin, Anne Nirupama, Colleen App, Renee Armour, Andrew Ashikari, Deanna Attai, Julie Barone, Darlene Barr, Katherine Barton, Peter Beitsch, Honnie Bermas, Tiffany Berry, Diane Bowers, Mindy Bowie, Elizabeth Brady, Eric Brown, Claire Buchanan, Erna Busch-Devereaux, David Carlson, Claire Carman, Joseph Casey, Jamie Caughran, Melita Charles, Joseph Contino, Kimberli Cox, Patricia Dawson, Michelle DeWing, Elizabeth Dupont, Firas Eladoumikdachi, Souzan El-Eid, Sheldon Feldman, Adora Fou, Leah Gendler, Nizar Habal, Ching Ho, Suzanne Hoekstra, Lisa Hopkins, Jenevieve Hughes, Melissa Hulvat, Marnie Kaplan, Pond Kelemen, Andrew Kenler, Patricia Kennedy, Jessica Keto, Alison Laidley, Camelia Lawrence, Laura Lazarus, Christine Lee, Pamela Li, Roberta Lilly, Suzanne Lynn, Robert Maganini, Donna-Marie Manasseh, Wendy Mikkelson, Alison Mishkit, Sunny Mitchell, Brenda Moorthy, Christine Moulds-Merritt, Mary Murray, Leigh Neumayer, Bridget Oppong, William Owens, Melody Paulishak, Catherine Plzak, Winnie Polen, Laura Pomerenke, Catherine Porter, Mary Pronovost, Stephen Ray, Adam Riker, David Rock, Margaret Sacco, Michael Schultz, Ingrid Sharon, Jeannie Shen, Paulomi Shroff, Diane Stoller, Walton Taylor, Kennith Thompson, Shannon Tierney, Judy Tjoe, Lisa Torp, Eleni Tousimis, John Turner, Gary Unzeitig, Aislinn Vaughan, Anna Voltura, Irene Wapnir, Barbara Ward, Barbara Wexelman, Eric Whitacre, Thomas Whitacre, Lisa Wiechmann, Shawna Willey, Brynn Wolff; (II) American Society of Breast Surgeons: Mena Jalai, Sharon Grutman, Margaret Schlosnagle, Marti Boyer, Ben Schlosnagle, and Jane Schuster; (III) Data analysis & interpretation: Biostat Inc. Maureen Lyden.
The ASBrS NSMR receives unrestricted educational grants from Invuity, Medtronic, and LifeCell.
Footnote
Conflicts of Interest: SD Mitchell receives honorarium and stock options from Invuity for her role on their Advisory Council and as Speaker and Consultant; SC Willey is on Speakers Bureaus for Pacira, Genentech, Invuity, and Medtronic, and on the Scientific Advisory Board of TransMed7; P Beitsch targeted Medical Education, Principle; S Feldman has no conflicts of interest to declare.
Ethical Statement: Participating surgeons routinely offer NSM, have performed at least 3 prior to registry participation, are enrolled in the Mastery of Breast Surgery Program, and in accordance with the following: IRB approval, ASBrS NSMR Protocol (v.3 07.2014), consent to act as a participant in the ASBrS NSMR, and Investigator Agreement. This assessment of data occurred at 74 months initiation of the Registry.
References
- Stolier AJ, Wang J. Terminal duct lobular units are scarce in the nipple: implications for prophylactic nipple-sparing mastectomy: terminal duct lobular units in the nipple. Ann Surg Oncol 2008;15:438-42. [Crossref] [PubMed]
- Rusby JE, Brachtel EF, Taghian A, et al. George Peters Award. Microscopic anatomy within the nipple: implications for nipple-sparing mastectomy. Am J Surg 2007;194:433-7. [Crossref] [PubMed]
- Petit JY, Veronesi U, Rey P., et al. Nipple-sparing mastectomy: risk of nipple-areolar recurrences in a series of 579 cases. Breast Cancer Res Treat 2009;114:97-101. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [Crossref] [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [Crossref] [PubMed]
- de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117-22. [Crossref] [PubMed]
- Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg 2011;212:686-93; discussion 693-5. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Howard MA, Sisco M, Yao K, et al. Patient satisfaction with nipple-sparing mastectomy: A prospective study of patient reported outcomes using the BREAST-Q. J Surg Oncol 2016;114:416-22. [Crossref] [PubMed]
- Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat 2009;118:623-33. [Crossref] [PubMed]
- Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009;62:586-90. [Crossref] [PubMed]
- Peled AW, Duralde E, Foster RD, et al. Patient-reported outcomes and satisfaction after total skin-sparing mastectomy and immediate expander-implant reconstruction. Ann Plast Surg 2014;72 Suppl 1:S48-52. [Crossref] [PubMed]
- Metcalfe KA, Cil TD, Semple JL, et al. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann Surg Oncol 2015;22:3324-30. [Crossref] [PubMed]
- Dossett LA, Lowe J, Sun W, et al. Prospective evaluation of skin and nipple-areola sensation and patient satisfaction after nipple-sparing mastectomy. J Surg Oncol 2016;114:11-6. [Crossref] [PubMed]
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 2015;22:370-6. [Crossref]
- Wang F, Peled AW, Garwood E, et al. Total skin-sparing mastectomy and immediate breast reconstruction: an evolution of technique and assessment of outcomes. Ann Surg Oncol 2014;21:3223-30. [Crossref] [PubMed]
- Li FC, Juang HC, Li J. Immediate breast reconstruction with implants after skin-sparing mastectomy: a report of 96 cases. Aesthetic Plast Surg 2010;34:705-10. [Crossref] [PubMed]
- Ota D, Fukuuchi A, Iwahira Y, et al. Identification of complications in mastectomy with immediate reconstruction using tissue expanders and permanent implants for breast cancer patients. Breast Cancer 2016;23:400-6. [Crossref] [PubMed]
- Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol 2013;20:3294-302. [Crossref] [PubMed]
- Al-Mufarrej FM, Woods JE, Jacobso SR. Simultaneous mastopexy in patients undergoing prophylactic nipple-sparing mastectomies and immediate reconstruction. J Plast Reconstr Aesthet Surg 2013;66:747-55. [Crossref] [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg 2014;134:396-404. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol 2013;108:207-12. [Crossref] [PubMed]
- Schneider LF, Chen CM, Stolier AJ, et al. Nipple-sparing mastectomy and immediate free-flap reconstruction in the large ptotic breast. Ann Plast Surg 2012;69:425-8. [Crossref] [PubMed]
- Chirappapha P, Petit JY, Rietjens M, et al. Nipple sparing mastectomy: does breast morphological factor related to necrotic complications? Plast Reconstr Surg Glob Open 2014;2:e99. [Crossref] [PubMed]
- Gunnarsson GL, Børsen-Koch M, Wamberg P, et al. How to perform a NAC sparing mastectomy using an ADM and an implant. Gland Surg 2014;3:252-7. [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Lee KT, Pyon JK, Bang SI, et al. Does the reconstruction method influence development of mastectomy flap complications in nipple-sparing mastectomy? J Plast Reconstr Aesthet Surg 2013;66:1543-50. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Salzberg CA. Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg 2012;130:95S-103S. [Crossref] [PubMed]
- Spear SL, Hannan CM, Willey SC, et al. Nipple-sparing mastectomy. Plast Reconstr Surg 2009;123:1665-73. [Crossref] [PubMed]
- Zhong T, McCarthy CM, Price AN, et al. Evidence-based medicine: breast reconstruction. Plast Reconstr Surg 2013;132:1658-69. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- Krishnan NM, Fischer JP, Basta MN, et al. Is Single-Stage Prosthetic Reconstruction Cost Effective? A Cost-Utility Analysis for the Use of Direct-to-Implant Breast Reconstruction Relative to Expander-Implant Reconstruction in Postmastectomy Patients. Plast Reconstr Surg 2016;138:537-47. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Tissue Expander Reconstruction After Total Skin-Sparing Mastectomy: Defining the Effects of Coverage Technique on Nipple/Areola Preservation. Ann Plast Surg 2016;77:17-24. [Crossref] [PubMed]
- Mendenhall SD, Anderson LA, Ying J, et al. The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast Reconstr Surg 2015;135:29e-42e. [Crossref] [PubMed]
- Casella D, Bernini M, Bencini L, et al. TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg 2014;37:599-604. [Crossref] [PubMed]
- Tessler O, Reish RG, Maman DY, et al. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg 2014;133:90e-9e. [Crossref] [PubMed]
- Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg 2016;137:385-93. [Crossref] [PubMed]
- Roostaeian J, Sanchez I, Vardanian A, et al. Comparison of immediate implant placement versus the staged tissue expander technique in breast reconstruction. Plast Reconstr Surg 2012;129:909e-18e. [Crossref] [PubMed]
- Levine SM, Snider C, Gerald G, et al. Buried flap reconstruction after nipple-sparing mastectomy: advancing toward single-stage breast reconstruction. Plast Reconstr Surg 2013;132:489e-97e. [Crossref] [PubMed]
- Tanna N, Broer PN, Weichman KE, et al. Microsurgical breast reconstruction for nipple-sparing mastectomy. Plast Reconstr Surg 2013;131:139e-47e. [Crossref] [PubMed]
- Raghavan S, Peled AW, Hansen SL, et al. Approaches to microvascular breast reconstruction after total skin-sparing mastectomy: a comparison of techniques. Ann Plast Surg 2015;74 Suppl 1:S46-51. [Crossref] [PubMed]
- Peled AW, Foster RD, Ligh C, et al. Impact of total skin-sparing mastectomy incision type on reconstructive complications following radiation therapy. Plast Reconstr Surg 2014;134:169-75. [Crossref] [PubMed]
- Dent BL, Cordeiro CN, Small K, et al. Nipple-Sparing Mastectomy via an Inframammary Fold Incision with Implant-Based Reconstruction in Patients with Prior Cosmetic Breast Surgery. Aesthet Surg J 2015;35:548-57. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Inframammary approach to nipple-areola-sparing mastectomy. Plast Reconstr Surg 2013;132:700e-8e. [Crossref] [PubMed]
- Huston TL, Small K, Swistel AJ, et al. Nipple-sparing mastectomy via an inframammary fold incision for patients with scarring from prior lumpectomy. Ann Plast Surg 2015;74:652-7. [Crossref] [PubMed]
- Colwell AS, Gadd M, Smith BL, et al. An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Ann Plast Surg 2010;65:140-3. [Crossref] [PubMed]
- Blechman KM, Karp NS, Levovitz C, et al. The lateral inframammary fold incision for nipple-sparing mastectomy: outcomes from over 50 immediate implant-based breast reconstructions. Breast J 2013;19:31-40. [Crossref] [PubMed]
- Maxwell GP, Storm-Dickerson T, Whitworth P, et al. Advances in nipple-sparing mastectomy: oncological safety and incision selection. Aesthet Surg J 2011;31:310-9. [Crossref] [PubMed]
- Wagner JL, Fearmonti R, Hunt KK, et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137-44. [Crossref] [PubMed]
- Ananthakrishnan P, Feldman S. Nipple-sparing mastectomy: indications, oncologic safety. Minerva Chir 2012;67:257-70. [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [Crossref] [PubMed]
- Stolier A, Stone JC, Moroz K, et al. A comparison of clinical and pathologic assessments for the prediction of occult nipple involvement in nipple-sparing mastectomies. Ann Surg Oncol 2013;20:128-32. [Crossref] [PubMed]
- Banerjee A, Gupta S, Bhattacharya N. Preservation of nipple-areola complex in breast cancer--a clinicopathological assessment. J Plast Reconstr Aesthet Surg 2008;61:1195-8. [Crossref] [PubMed]
- Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol 2009;27:4948-54. [Crossref] [PubMed]
- Rusby JE, Brachtel EF, Othus M, et al. Development and validation of a model predictive of occult nipple involvement in women undergoing mastectomy. Br J Surg 2008;95:1356-61. [Crossref] [PubMed]
- Loewen MJ, Jennings JA, Sherman SR, et al. Mammographic distance as a predictor of nipple-areola complex involvement in breast cancer. Am J Surg 2008;195:391-4; discussion 394-5. [Crossref] [PubMed]
- Wijayanayagam A, Kumar AS, Foster RD, et al. Optimizing the total skin-sparing mastectomy. Arch Surg 2008;143:38-45; discussion 45. [Crossref] [PubMed]
- Crowe JP Jr, Kim JA, Yetman R, et al. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg 2004;139:148-50. [Crossref] [PubMed]
- Crowe JP, Patrick RJ, Yetman RJ, et al. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch Surg 2008;143:1106-10; discussion 1110. [Crossref] [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [Crossref] [PubMed]
- Voltura AM, Tsangaris TN, Rosson GD, et al. Nipple-sparing mastectomy: critical assessment of 51 procedures and implications for selection criteria. Ann Surg Oncol 2008;15:3396-401. [Crossref] [PubMed]
- Coopey SB, Smith BL. The Nipple is Just Another Margin. Ann Surg Oncol 2015;22:3764-6. [Crossref] [PubMed]


