Thyroid nodules coexisting with either cystic or solid breast nodules: a new clue for this association between nodules coming from ultrasonography
Introduction
Thyroid nodules (TNs) are common, their prevalence varying based mainly on the population (including age, residence in area of different iodine availability) studied and methods used (1). The prevalence is greater in women compared with men, in the elderly compared with adults and children/adolescents, in iodine-deficient areas compared to iodine-sufficient areas, and in persons exposed to radiation compared to non-exposed persons (1). Concerning the methodology for detecting TNs, the reported prevalence ranges from 2% to 6% with palpation, from 19% to 35% with ultrasonography (US), and from 8% to 65% at autopsy (1). With the widespread use of sensitive imaging in clinical practice, incidental TNs are being discovered with increasing frequency (1). US is the most accurate and cost-effective method for the detection and initial evaluation of TNs (1,2).
Breast nodules (BNs) are also common, their prevalence varying based mainly on the population studied (including age and family history of breast cancer) and methods used (3,4). The prevalence is greater in women aged 40–44 years than in those aged 25–29 years, and in women with a family history of breast cancer (3). Concerning the methodology for detecting BNs, the prevalence was reported to range from 54% to 92% using palpation (5), from 50% to 90% using US and from 46% to 86% at autopsy (6,7). Refinement of high-frequency technology, particularly the 7.5–13 MHz probes, has brought out a totally new facet in US breast imaging (8). Harmonic imaging and real-time compounding improve image resolution and lesion characterization (9). More recently, the use of US elastography can improve the specificity and positive predictive value of US in the characterization of breast masses (4). US elastography is useful in discriminating between benign and malignant solid masses (10,11), and it is superior to mammography in diagnosing clinically benign palpable masses (up to 97% accuracy vs. 87%) (11). Similar to TNs, the vast majority of BNs are benign (12).
The coincidence of thyroid diseases (TD) and breast diseases remains controversial; the majority of the studies have evaluated the association between breast cancer and thyroid disorders (13-26).
In the clinical setting of an ambulatory US service, we wished to ascertain: (I) the rate of request of both thyroid and breast US; (II) the characteristics of both the TNs and BNs detected by US. The latter aim was because it was our recent impression that certain characteristics of TN are different based on the cystic or solid nature of the BN (CBN or SBN).
Methods
Study population
Between the years 2000 and 2005, 3,372 patients from outpatient clinics were referred for thyroid US, 2,235 of whom were found to have one or more TNs (cystic and/or non-cystic ones). Among these 2,235 patients, 1,896 (84.8%) were females and 339 (15.2%) males (age: 66.1±11.8 years for women and 61.3±12.3 years for men); an association with extrathyroidal nodules was searched based on US examinations of other organs. In 391 cases, such association was found (336 or 85.9% F and 55 or 14.1% M). Concurrent breast US in the 1,896 women revealed the presence of BNs in 127. The 127 women were divided into two groups, based on the cystic or solid nature of the BN(s). In 84/127 (66.1%) the single or multiple BN was/were cystic (group 1), whereas in the remaining 43 (33.9%) the single or multiple BN was/were solid (group 2; 19/43 had 1 TN and the remaining 24/43 had ≥2 TNs). Patients with fibrocystic breast disease or changes were excluded.
Thyroid US
Thyroid US was performed at the Oncology Division, with a Logiq by General Electric (Boston, MA, USA) instrumentation equipped with a 7.5–10.0 MHz linear transducer. The thyroid parameters evaluated were volume, echo-texture, vascularization and nodules. Thyroid echo-texture is a fundamental feature to judge if thyroid inflammation is present. If inflammation exists, regardless of thyroid volume, the pattern is one of diffuse and dyshomogeneous hypoechogenicity with or without pseudonodules. The number, maximum diameter and ultrasonographic characteristics of all nodules were noted. As classically done, TNs were defined micronodules if their maximum diameter was 10 millimeters or smaller. Thyroid pseudonodules, which are encountered frequently in an inflamed thyroid, were not counted as nodules. The results of thyroid US were classified as follows: (I) absence of cysts and nodules; (II) presence of cysts; (III) presence of nodules. In cases of multiple cysts or nodules, we considered the maximum diameter of the largest lesion.
Breast US
Breast US was performed at the Oncology division, with a Logiq by General Electric instrumentation equipped with a 7.5–10.0 MHz linear transducer. A cyst is seen on US as a well-defined, round or oval, anechoic structure with a thin wall. In contrast, solid structures were considered nodules. If internal echoes or debris are seen in a cyst, the cyst is called a complex cyst. These internal echoes may be caused by floating cholesterol crystals, pus, blood or milk of calcium crystals (7).
Statistics
Differences between proportions of categorical variables were handled by the chi-squared test or the Fisher’s exact test, as appropriate. For any statistical comparison, which was always two-tailed, a P value of less than 0.05 was considered statistically significant and P values comprised between 0.05 and 0.10 were considered borderline significant.
Results
Age
Considering the cystic BNs (CBNs) (group 1), the decades at US most represented were the fifth (41–50 years) and the fourth (31–40 years), in that they accounted for 53.6% and 20.2%, respectively, of all women in group 1. A similar distribution was observed for the solid BNs (group 2), namely 51.1% and 16.2%, with no intergroup statistical difference (Df =6, χ2 =8.12, P=0.229).
Localization of the thyroid and BNs.
Table 1 shows the 3-tier localization of nodules (right, left, bilateral), with lateralization applying to the lobe for the thyroid (right lobe, left lobe or both) and the breasts for the mammary nodules (right breast, left breast or both). In the 84 women with CBN, the bilateral localization was the predominant one for both the BN and TN (59.5% and 57.1%, respectively). In contrast, in the 43 women with solid BN, the bilateral localization predominated for the TN (58.1%) but not for the BN, in which the leading localization was the right one (39.5%). As a consequence, distribution of the BN and TN among the three sites did not differ significantly in group 1 women (χ2 =1.49, P=0.47), but it did so in group 2 women (χ2 =6.52, P=0.038) (Table 1). From another perspective, distribution of TN among the three sites did not differ significantly between group 1 and group 2 women (χ2 =1.35, P=0.51), but it did so the distribution of the BN (χ2 =9.20, P=0.01) (Table 1).

Full table
Figure 1 summarizes all the possible combinations in localization of the TN and BN, distinguishing between the 21 women with a single TN (top panel) and the 63 with two or more TNs (bottom panel). Consistently, when BN were cystic the coexisting thyroid nodule/nodules was/were more likely to be concordant in localization (Figure 1, top and bottom panels). In contrast, when the BNs were solid, the coexisting thyroid nodule/nodules was/were more likely to be discordant (Figure 1, top and bottom panels).
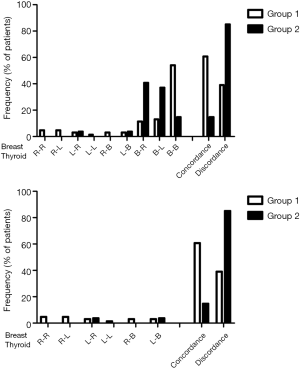
Number and localization of TN
In the 84 patients of group 1, 200 (or 2.4 per patient) TNs were found: patients with 1, 2 and ≥3 nodules were, respectively, 36.9%, 19% and 44%. Considering the localization of the TNs in the two lobes, the classic topography in the upper, middle or lower third (or area or region) was used. Either for the 27 cases with TNs (cystic and/or non-cystic ones) in the left lobe or for the 38 cases in the right lobe, the most represented localization was the lower third which accounted for 20 (74.1%) and 26 (68.4%) cases, respectively. Among the patients with single TN, both for the 9 cases with cystic or non-cystic TN in the left lobe or for the 14 cases in the right thyroid lobe, the most represented was the lower third localization which accounted, respectively, for 6 (66.7%) and 8 (57.1%) cases.
In the 43 patients of group 2, 86 (or 2 per patient) TNs were found: patients with 1, 2 and ≥3 nodules were, respectively, 48.8%, 20.9% and 30.2% (Df =2, χ2 =2.419, P=0.298 vs. group 1). Considering the localization of the TN in the two lobes, the classic topography in the upper, middle or lower third (or area or region) was used. For the 26 cases with TNs (cystic and/or non-cystic ones) in the left lobe, the most represented localization was the inferior third, whereas for the 17 cases in the right lobe the most represented localization was the middle third. Among the 13 patients with single TN, both middle and inferior third were equally involved in the left lobe (50% of cases, both) (P=0.58 by Fisher’s exact test vs. group 1), whereas the middle third localization prevailed for the right thyroid lobe.
TN echogenicity
Evaluating echogenicity of TNs in patients of group 1, hypoechoic TNs were the more frequent ones (76 cases or 90.5%). Based on the total number of TNs (n=203), hypoechoic TNs remained the most frequent (n=151 or 74.4%). Also among the 23 patients with single cystic or non-cystic TN associated with breast cysts, hypoechoic TNs were the most frequent ones (19 cases or 82.6%).
Considering echogenicity of TNs in patients of group 2, hypoechoic TNs were the most frequent ones (39 cases or 90.7%) (Df =3, χ2 =0.568, P=0.904 vs. group 1). Based on the total number of TNs (n=66), hypoechoic TNs remained the most frequent (n=54 or 81.8%) (Df =3, χ2 =1.439, P=0.697 vs. group 1). Also among the 13 patients with single cystic or non-cystic TN associated with SBN, hypoechoic TNs were the most frequent ones (11 cases or 84.6%). Overall, in group 2, the BN had US features of fibroma (n=32), lipoma (n=4) or undefined (n=7).
TNs: maximum diameter
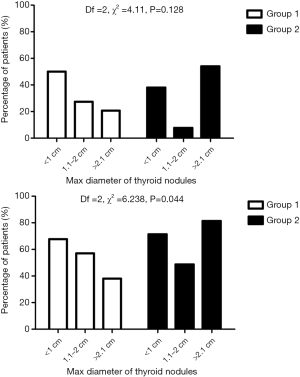
Figure 2 shows the 3-tier stratification of single TN (top panel) or multiple TN (bottom panel) based on their maximum diameter. Consistently, when BN were cystic, the coexisting thyroid nodule/nodules was/were more likely to be subcentimetric and less likely to be >2.1 cm in maximum diameter (top and bottom panels). In contrast, when BN were solid, the coexisting thyroid nodule/nodule was/were more likely to be >2.1 cm and less likely to be 1.1–2.0 cm in maximum diameter (top and bottom panels). Because of a smaller number of women with a single TN in group 1 (n=31) and group 2 (n=19), only the 3-tier partition of multiple TN was significantly different in group 1 compared to group 2 women (Df =2, χ2 =6.238, P=0.044) (Figure 2).
Discussion
We here evaluated the rate with which, at an ambulatory facility for US located in a tertiary care medical center, mammary US is requested as an ancillary exam for a woman whose primary referral reason is thyroid US. We also evaluated the characteristics of the TNs, in light of the cystic or solid nature of the coexisting BNs. Indeed, based on few women observed at the endocrine unit of the same university hospital (see Introduction), some different features depending on the nature (cystic or solid) of the BNs are present in the same woman.
We found that 6.7% (127/1,896) of the women referred for the thyroid US also had undergone a mammary US. BNs were cystic in two-thirds of women (84/127), and solid in the remaining one-third (43/127).
Limitations of the study are the retrospective nature and the lack of cytological diagnosis for those nodules that deserved fine-needle aspiration biopsy (FNAB). Another limitation could be that we have not looked at the opposite relationship, viz. request of thyroid US in women referred primarily for breast US.
In women with CBNs, TNs were more frequently multiple (64% of cases), whereas in cases with solid BNs, the frequency of single and multiple TNs was approximately the same. The nature (cystic or solid) of the single or all nodules present in the breast/s somehow influenced certain characteristics of the coexisting thyroid nodule/s. When the coexisting breast nodule(s) was/were cystic, the coexisting nodules most frequently detected in the thyroid were the subcentimetric ones (approximately 70% of all TNs). This rate of the subcentimetric TNs and that of the TNs 1.1–2.0 cm in maximum diameter (approximately 55% of all TNs) held when the coexisting breast nodule/s was/were solid. In contrast, the rate of the largest TNs doubled from the approximate 40% in women with cystic breast nodule/s to approximately 80% in women with solid breast nodule(s). This pattern for the largest TNs held when solitary TNs were considered, and in this case, it was evident that such increase occurred at the expense of the other categories (<1.0 and 1.1–2.0 cm).
Excluding the particular association of BN and TN as consequence of breast malignancy that metastasizes to the thyroid (13-26) and a study from Spinos et al. (27) which determined cross-sectionally the incidence of solitary TNs and breast fibroadenomas in women with uterine fibroids, the concomitance of TD and breast disease remains controversial. Chung et al. (28) conducted a study to clarify the sensitivity of ultrasonographic mass screening for thyroid carcinoma and evaluated 1,401 women who were scheduled to undergo either a breast examination or a follow-up examination for breast cancer. They found that US mass screening for thyroid carcinoma in women who require breast examinations was effective for the detection of subclinical thyroid carcinoma. TNs were detected in 353 (25.2%) of women, 94 (26.6%) of whom were placed in the high-risk group for thyroid cancer, based on suspicious US characteristics and subsequent FNAB on high-risk patients. Among the 94 high-risk patients, 43 underwent thyroidectomy and 37 turned out to have thyroid carcinoma. The detection rate for thyroid cancer was 2.6% (37/1,401). Giani et al. (29) provided evidence that the overall prevalence of thyroid disorders is increased in patients with breast cancer. Particularly, Hashimoto’s thyroiditis, account to a large extent for the increased prevalence of TD in patients with breast cancer (29). Sun et al. (30) analyzed 55,318 Taiwanese women with breast cancer of whom 28,187 received radiotherapy and 27,131 did not. In that study, only younger (age 20–54 years) women with breast cancer were found to have a significantly higher risk of developing thyroid cancer. Utilizing the surveillance, epidemiology, and End Results-9 database in a retrospective cohort analysis on women older than age 18 years with breast and thyroid cancer, Kuo and colleagues (31) concluded that thyroid cancer survivors are at greater risk for developing breast cancer than the general population. A recent systematic review and meta-analysis including six cohort studies with 17,914 patients found that the relative risk of second primary breast cancer in thyroid cancer survivors treated with radioactive iodine was 0.61 relative to thyroid cancer survivors not treated with radioactive iodine, therefore not increased (32). Interestingly, gene expression profiling analysis of chemokine receptors CXCR7 and CXCR4 in patients with breast cancer and in patients with papillary thyroid cancer showed a role of the chemokine receptors in tumor progression (33,34).
Regulatory subunits of protein kinase A (PKA) are important in cell growth and cell differentiation. PKA hyperactivation has been shown to drive mammay tumorigenesis (35) and increased expression of regulatory subunit R1A of PKA is associated with aggressive and undifferentiated thyroid tumors (36). Another important factor in the pathogenesis of thyroid and BNs and cancer is the environmental exposure to certain chemicals including polychlorinated dibenzo-p-dioxins and dibenzofurans and derivates (37-39). These chlorinated aromatic compounds are highly soluble in fat and oils and therefore preferentially deposited in such tissues (40).
In 2011, Muller et al. (41) evaluated the prevalence of breast cancer in a large group of patients with benign TD and found that the breast cancer prevalence in patients with benign TD was significantly higher compared to BC frequency in the general population, showing the usefulness of screening for breast malignancy of patients with benign TD. Prinzi et al. (42) analyzed the prevalence of extra-thyroidal malignancies (EM) in 6,386 female patients affected by different TD. At first, an age-matched analysis of EM in all patients was performed. We then evaluated EM prevalence in four TD diagnostic categories: non-nodular TD (n=2,159); solitary nodule (n=905); multinodular TD (n=2,871); differentiated thyroid cancers (n=451). A total of 673 EM were recorded. EM prevalence in TD patients was higher compared to the general population [odds ratio (OR) 3.21] and the most frequent EM was breast cancer (OR 3.94). Breast cancer showed an increased OR in all TD, while other cancers associated with specific TD. Women affected by both benign and malignant TD, especially at a younger age, were found to have an increased risk of developing primary EM, thus requiring a careful follow-up and surveillance. Recently, some studies have demonstrated an association between thyroid and breast cancer (43,44). In particular, not only women with a prior history of differentiated thyroid cancer are at an increased risk for breast cancer, but also women with a history of breast cancer are at an increased risk for differentiated thyroid cancer (44).
To the best of our knowledge, studies in the non-oncological literature assessing the coexistence between TNs and BNs have not yet been performed.
In conclusion, in women with BNs associated with TNs, there are interesting differences concerning TNs when patients are stratified based on the cystic or solid nature of the BN. In patients with TNs/CBNs, TNs tend to be subcentimetric and localized in the inferior pole of either thyroid lobe. In patients with TNs/SBNs, TNs tend to be larger and localized in the middle of either thyroid lobe. Our data raise the intriguing possibility that the thyroid follicular epithelium (TFE) positioned along the longitudinal axis of the thyroid gland has differential sensitivity to hypothetical substances, that are produced by CBNs and SBNs and that exert hyperplastic effects on TFE. CBN-secreted substance(s) is/are likely to be distinct from SBN-secreted substance(s), to the hyperplastic stimulus of which the TFE of the inferior pole is refractory.
Frequent performance of thyroid and breast US over many years is required in order to establish which nodule(s) (thyroid or breast) appeared first, and how it/they may influence the US characteristics of the nodule/nodules of the other tissue.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Notwithstanding the retrospective nature of this original article, the local Ethics Committee (University Hospital of Messina) approved it by giving the number E-49/99. Informed consent was obtained from all individual participants included in the study.
References
- Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab 2008;22:901-11. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Webb PM, Byrne C, Schnitt SJ, et al. Family history of breast cancer, age and benign breast disease. Int J Cancer 2002;100:375-8. [Crossref] [PubMed]
- Zhi H, Ou B, Luo BM, et al. Comparison of Ultrasound Elastography, Mammography, and Sonography in the Diagnosis of Solid Breast Lesions. J Ultrasound Med 2007;26:807-15. [Crossref] [PubMed]
- Dayan A. Frequency of Clinically Palpable Lumps in Patients Presenting with Breast Disease in Breast Clinic. JSOGP 2013;3:212-7.
- Rinaldi P, Ierardi C, Costantini M, et al. Cystic breast lesions: sonographic findings and clinical management. J Ultrasound Med 2010;29:1617-26. [Crossref] [PubMed]
- Bhathal PS, Brown RW, Lesueur GC, et al. Frequency of benign and malignant breast lesions in 207 consecutive autopsies in Australian women. Br J Cancer 1985;51:271-8. [Crossref] [PubMed]
- Catarzi S, Giuseppetti GM, Rizzatto G, et al. A multicenter study for the evaluation of the diagnostic efficiency of mammography and echography in nonpalpable breast neoplasms. Radiol Med 1992;84:193-7. [PubMed]
- Merritt CR. Technology update. Radiol Clin North Am 2001;39:385-97. [PubMed]
- Moss HA, Britton PD, Flower CD, et al. How reliable is modern breast imaging in differentiating benign from malignant breast lesions in the symptomatic population? Clin Radiol 1999;54:676-82. [Crossref] [PubMed]
- Lister D, Evans AJ, Burrell HC, et al. The accuracy of breast ultrasound in the evaluation of clinically benign discrete, symptomatic breast lumps. Clin Radiol 1998;53:490-2. [Crossref] [PubMed]
- Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist 2006;11:435-49. [Crossref] [PubMed]
- Agarwal DP, Soni TP, Sharma OP, et al. Synchronous malignancies of breast and thyroid gland: a case report and review of literature. J Cancer Res Ther 2007;3:172-3. [Crossref] [PubMed]
- Jeong YJ, Oh HK, Bong JG. Multiple endocrine neoplasia type 1 associated with breast cancer: A case report and review of the literature. Oncol Lett 2014;8:230-4. [PubMed]
- Park LC, Jeong JY, Ji JH, et al. Intra-tumoral Metastatic Double Primary Carcinoma: Synchronous Metastatic Tumor in Lung from Breast and Thyroid Carcinoma. Cancer Res Treat 2014;46:200-3. [Crossref] [PubMed]
- Liu YP, Tiu CM, Chou YH, et al. Thyroid metastasis from breast cancer presenting with diffuse microcalcifications on sonography: A case report. J Clin Ultrasound 2014;42:430-2. [Crossref] [PubMed]
- Nguyen MS, Ginat DT, Giampoli EJ, et al. Breast metastases to thyroid gland. Ultrasound Q 2013;29:327-8. [Crossref] [PubMed]
- Zhou XC, Hu KQ, Zhang XF, et al. Breast cancer metastatic to the bilateral thyroid: A case report and literature review. Head Neck Oncol 2012;4:51.
- Lacka K, Breborowicz D, Uliasz A, et al. Thyroid metastases from a breast cancer diagnosed by fine-needle aspiration biopsy. Case report and overview of the literature. Exp Oncol 2012;34:129-33. [PubMed]
- Zeng H, Liu C, Zeng YJ, et al. Collision metastasis of breast and thyroid carcinoma to a single cervical lymph node: report of a case. Surg Today 2012;42:891-4. [Crossref] [PubMed]
- Cihan YB, Deniz K, Yilmaz MS. Synchronous Parotid and Thyroid Gland Metastases from Breast Cancer. Breast Care (Basel) 2011;6:133-5. [Crossref] [PubMed]
- Owens CL, Basaria S, Nicol TL. Metastatic breast carcinoma involving the thyroid gland diagnosed by fine-needle aspiration: a case report. Diagn Cytopathol 2005;33:110-5. [Crossref] [PubMed]
- Feldman MD, Baloch ZW. LiVolsi VA. Metastatic breast carcinoma mimicking primary thyroid neoplasm in young women. Endocr Pract 1999;5:343-6. [Crossref] [PubMed]
- Jiménez-Heffernan JA, Perez F, Hornedo J, et al. Massive thyroid tumoral embolism from a breast carcinoma presenting as acute thyroiditis. Arch Pathol Lab Med 2004;128:804-6. [PubMed]
- Huang J, Walker R, Groome PG, et al. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer 2001;92:1411-8. [Crossref] [PubMed]
- Ferrara G, Ianniello GP, Nappi O. Thyroid metastases from a ductal carcinoma of the breast. A case report. Tumori 1997;83:783-7. [PubMed]
- Spinos N, Terzis G, Crysanthopoulou A, et al. Increased frequency of thyroid nodules and breast fibroadenomas in women with uterine fibroids. Thyroid 2007;17:1257-9. [Crossref] [PubMed]
- Chung WY, Chang HS, Kim EK, et al. Ultrasonographic mass screening for thyroid carcinoma: a study in women scheduled to undergo a breast examination. Surg Today 2001;31:763-7. [Crossref] [PubMed]
- Giani C, Fierabracci P, Bonacci R, et al. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab 1996;81:990-4. [PubMed]
- Sun LM, Lin CL, Liang JA, et al. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: a nationwide population-based cohort study. Int J Cancer 2015;137:2896-903. [Crossref] [PubMed]
- Kuo JH, Chabot JA, Lee JA. Breast cancer in thyroid cancer survivors: an analysis of the surveillance, epidemiology, and end results-9 database. Surgery 2016;159:23-9. [Crossref] [PubMed]
- Zhang Y, Liang J, Li H, et al. Risk of second primary breast cancer after radioactive iodine treatment in thyroid cancer: a systematic review and meta-analysis. Nucl Med Commun 2016;37:110-5. [Crossref] [PubMed]
- Wu W, Qian L, Chen X, et al. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int J Clin Exp Pathol 2015;8:13217-24. [PubMed]
- Zhang H, Teng X, Liu Z, et al. Gene expression profile analyze the molecular mechanisms of CXCR7 regulating papillary thyroid carcinoma growth and metastasis. J Exp Clin Cancer Res 2015;34:16. [Crossref] [PubMed]
- Beristain AG, Molyneux SD, Joshi PA, et al. PKA signaling drives mammary tumorgenesis through Src. Oncogene 2015;34:1160-73. [Crossref] [PubMed]
- Ferrero S, Vaira V, Del Gobbo A, et al. Different expression of protein kinase A (PKA) regulatory subunits in normal and neoplastic thyroid tissues. Histol Histopathol 2015;30:473-8. [PubMed]
- Mitra AK, Faruque FS. Breast cancer incidence and exposure to environmental chemicals in 82 counties in Mississippi. South Med J 2004;97:259-63. [Crossref] [PubMed]
- Verkasalo PK, Kokki E, Pukkala E, et al. Cancer risk near a polluted river in Finland. Environ. Health Perspect 2004;112:1026-31. [Crossref] [PubMed]
- Ruder AM, Yiin JH. Mortality of US pentachlorophenol production workers through 2005. Chemosphere 2011;83:851-61. [Crossref] [PubMed]
- Hardell L, Lindstrom G, Liljegren G, et al. Increased concentrations of octacholrodibenzo-p-dioxine in cases with breast cancer-results from a case-control study. Eur J Cancer Prev 1996;5:351-7. [Crossref] [PubMed]
- Muller I, Pinchera A, Fiore E, et al. High prevalence of breast cancer in patients with benign thyroid diseases. J Endocrinol Invest 2011;34:349-52. [Crossref] [PubMed]
- Prinzi N, Sorrenti S, Baldini E, et al. Association of thyroid diseases with primary extra-thyroidal malignancies in women: results of a cross-sectional study of 6,386 patients. PLoS One 2015;10:e0122958. [Crossref] [PubMed]
- An JH, Hwangbo Y, Ahn HY, et al. A Possible Association Between Thyroid Cancer and Breast Cancer. Thyroid 2015;25:1330-8. [Crossref] [PubMed]
- Nielsen SM, White MG, Hong S, et al. The Breast-Thyroid Cancer Link: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev 2016;25:231-8. [Crossref] [PubMed]


