Incidence, prevalence and risk factors for post-surgical hypocalcaemia and hypoparathyroidism
Introduction
Post-surgical hypoparathyroidism (PoSH) manifests as hypocalcaemia and may be due to damage to parathyroid blood supply and/or inadvertent excision of the gland (1). Another mechanism for temporary post-surgical hypocalcaemia includes hungry bone syndrome where there is rapid and acute shift of calcium into the bones following surgical treatment of patients with preoperative thyrotoxicosis (2). Other postulated reasons include dilutional hypocalcaemia (3) and calcitonin release (4) following surgery; but empiric evidence of these phenomena as significant causes of post-surgical hypocalcaemia are lacking.
Acute hypocalcaemia may present with paraesthesia and neuromuscular instability. Long term hypocalcaemia is associated with abnormal renal function, cataracts, ectopic calcifications, seizures, abnormal teeth and psychiatric illnesses (5,6).
There is currently no international consensus on the definition of PoSH, resulting in many different definitions mentioned in the literature (7). The British Association of Endocrine and Thyroid Surgeons (BAETS) defines postoperative hypocalcaemia as day one postoperative calcium of less than 2.1 mmol/L (8). Late hypocalcaemia is defined as the need of calcium and/or vitamin D supplement to maintain normocalcaemia at 6 months or more following surgery (8). The European Society of Endocrinology considers chronic PoSH in patients with a low calcium and inappropriately low intact parathyroid hormone (iPTH) at more than 6 months (9). No specific serum levels of calcium or iPTH levels were given. A recent review by the American Association of Clinical Endocrinologist (AACE) and American College of Endocrinology (ACE) discussed definitions and management of PoSH (10). Hypoparathyroidism was categorised as biochemical and clinical; temporary and permanent. The distinction between temporary and permanent PoSH was made at 12 months. Relative hypoparathyroidism was also recognised as an entity where biochemical parameters were normal but medication was required for symptomatic relief. In terms of postsurgical hypocalcaemia, the same duration was applied to distinguish between temporary and permanent conditions and a total serum calcium of less than 8.5 mg/dL (2.125 mmol/L) or ionised calcium less than 1.15 mmol/L were considered as cut-off levels. In recent literature, ‘protracted hypoparathyroidism’ has been used to identify patients with persisting hypoparathyroidism at 4–6 weeks of surgery (1,11). This duration is in between those used for immediate or temporary and the long term definitions and is used in studies evaluating relatively short term outcomes.
The lack of inclusion of patients that need calcium and/or vitamin D supplements despite their PTH levels being in the normal range in some of the definitions above risk underestimating the true incidence and prevalence of this problem in the community.
Although PoSH may occasionally be a result of other operative procedures such as parathyroid surgery (12) and laryngeal surgery with concomitant thyroidectomy (13), the focus of this review is primarily on post-thyroidectomy hypocalcaemia and/or hypoparathyroidism. The terms hypocalcaemia and hypoparathyroidism are both used in this paper—this is to accurately reflect the definitions used in the original articles and reviews cited in this article. Although there is controversy over whether the words ‘long term’ or ‘permanent’ is most appropriate; both are used interchangeably in the article to reflect the descriptions ueds in the cited references. This highlights the need for a consensus statement on the definitions of PoSH.
Incidence
The incidence of PoSH varies significantly, partly as a result of difference in definitions used. Mehanna et al. found that the incidence rate ranges from 0% to 46% for the same cohort of patients depending on the definition used (7). Parameters used in the literature to define temporary postsurgical hypocalcaemia includes calcium level alone; calcium level and symptoms; calcium level and the need for treatment; calcium level, symptoms and need of treatment; symptoms alone; symptoms; and need for treatment and PTH level (14). To define long term or permanent postsurgical hypocalcaemia and/or hypoparathyroidism, studies have used calcium level alone; calcium level and duration; need for treatment and duration; symptoms and duration; calcium level, symptoms and duration; calcium level, PTH, need for treatment and duration; PTH level and duration; and PTH level, need for treatment and duration (14). Most studies used a duration of six month or more to define long term hypocalcaemia (14).
In a systematic review and meta-analysis of 115 studies, the median (IQR) incidence of temporary and long term post-surgical hypocalcaemia was 27% (19–38%) and 1% (0–3%) respectively (15). However, the studies used different definitions and are therefore not comparable. The fifth BAETS audit reported temporary and late hypocalcaemia rate of 23.6% (95% CI, 22.7–24.5%) and 6.5% (95% CI, 5.8–7.2%) respectively in patients who had total thyroidectomy (8). However, 10.1% and 21.5% of patients had data missing for temporary and long term outcomes respectively. A large multicentre Scandinavian audit reported 6 month hypocalcaemia rate of 4.4% following bilateral thyroid surgery. However, missing data occurred in 4.5% of patients (16).
In the United Kingdom (UK) and elsewhere, some centres routinely give prophylactic calcium and vitamin supplements to patients undergoing bilateral thyroid surgery (8). This can distort the incidence rates of post-surgical hypocalcaemia; especially when the definitions are based on serum calcium level. In a meta-analysis, the use of routine prophylactic calcium and vitamin D supplements (compared to oral calcium only or no supplements) reduced the incidence of temporary hypocalcaemia (14). In a randomised control trial (RCT) of 120 patients, use of calcium and vitamin D supplements reduced symptomatic hypocalcaemia when compared to oral calcium alone (17). However, routine use of supplementations did not impact on the rates of long term hypocalcaemia (14).
Prevalence
Few population studies have examined the prevalence of PoSH.
A Danish population based study (between 1988 and 2012) estimated the prevalence of PoSH using historic data from the Danish National Patient Registry. Patients were identified through the Danish National Patient Registry and Prescription database (18). Long term PoSH was defined as hypocalcaemia with inappropriately low PTH following neck surgery that required treatment with calcium and/or vitamin D supplements for more than 6 months. They identified a total of 980 patients with PoSH; 688 (70%) due to surgery for malignant disease and 292 (30%) for non-malignant disease. The estimated prevalence of long term PoSH in patients who had surgery for non-malignant disease was 22 per 100,000 (18).
Another study from the United States of America estimated the ‘diagnosis-based’ prevalence of hypoparathyroidism using a large insurance claims database (19). They identified all cases of hypoparathyroidism over a 12-month period (October 2007 and September 2008) and then extrapolated it to the insured US population. The prevalence of chronic hypoparathyroidism (not resolving within 6 months) in the insured US population in the surgical database was 58,793. Assuming that the US population in 2008 was 308.7 million and 82% are insured (20), this translates to a long term prevalence of PoSH of 23 per 100,000.
Astor et al. examined the prevalence of PoSH in Norway using an electronic hospital registry. They captured around 80% of the Norwegian population. Long term PoSH was defined as low serum calcium and inappropriately low PTH, necessitating treatment for more than one year. The reported prevalence of long term PoSH was 64 per million (21).
The incidence and prevalence of PoSH is likely to increase given the rising numbers of thyroid surgery being performed (22). This is in keeping with the sustained and global increase in diagnosis of thyroid cancer over the past few decades (23-25).
A number of biochemical, surgical related and patient/disease related factors have been identified as predictors of postoperative hypocalcaemia. These are listed in Table 1.
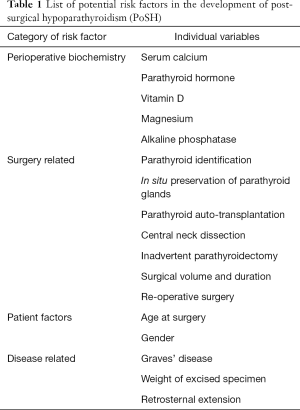
Full table
Biochemical predictors of hypoparathyroidism
Serum calcium
A low preoperative serum calcium level could potentially increase the likelihood and severity of postoperative hypocalcaemia. The association between preoperative serum calcium levels and post-operative hypocalcaemia is unclear. A large multicentre Scandinavian study of 3,660 patients found that a lower level of preoperative serum calcium was associated with postoperative hypocalcaemia (16). However, no cut off was specified to aid decision-making. In contrast, a recent meta-analysis including 2,493 patients from six studies found no association between preoperative serum calcium levels and postoperative hypocalcaemia (15). However, it would appear reasonable to check calcium levels preoperatively and in those with hypocalcaemia, evaluate and treat accordingly.
Some centres measure changes in calcium levels after surgery (‘calcium slope’) to predict PoSH and direct management of patients. A positive calcium slope within 24 hours of surgery (i.e., a rise in calcium between two consecutive measurement) has a positive predictive value (PPV) of 86–100% for excluding temporary postoperative hypocalcaemia (15). Pattou et al. examined predictors of permanent hypocalcaemia (at 12 months post-thyroidectomy) in 1,071 consecutive patients who underwent total or subtotal thyroidectomy (26). In patients receiving calcium supplementations at 1 to 3 weeks postoperative, they found that serum calcium of 2 mmol/L or lower significantly increased the risk of permanent hypocalcaemia in univariate analysis. Another study of 453 compared serum calcium levels between patient who had temporary and permanent hypocalcaemia. At one week post-operatively, 2 out of 44 patients in the temporary group had calcium lower that 2 mmol/L compared to 2 out of 3 in permanent group (P=0.001) (27). Both these studies were limited by the small numbers of patients who developed permanent hypocalcaemia.
In addition to their role in predicting the likelihood of long term hypocalcaemia and/or hypoparathyroidism, postoperative calcium levels are mainstay for the detection and appropriate treatment of hypocalcaemia.
PTH
Perioperative PTH measurements are used in many centres to predict post-operative hypocalcaemia. Studies have shown patients with low intraoperative PTH (any time from resection of the gland up to 10 minutes after resection), low postoperative PTH levels (30 minutes to 5 days after surgery), and decline of PTH between preoperative and postoperative measurements can be used to predict temporary post-thyroidectomy hypocalcaemia (15).
The utility of 4-hour iPTH and perioperative iPTH decline was studied in a prospective study of 137 patients who underwent total thyroidectomy (28). Decline in iPTH levels (preoperative to 4 hours postoperatively) of 68.5% or more from preoperative levels was more accurate (PPV: 90.5%) compared to 4-hour iPTH (PPV: 82.5%) in predicting postoperative hypocalcaemia. In another prospective study of 106 patients, iPTH decline of 80% or more at 3 hours after surgery was found to have a PPV of 100% for postoperative hypocalcaemia (29).
In the subgroup of patients who had postsurgical PoSH, undetectable serum iPTH (compared to low but detectable) at 4 weeks after surgery was an independent predictor of permanent PoSH. In the group with undetectable PTH (n=34), 13/34 (38.2%) had permanent PoSH (1).
A prospective study of 70 patients examined the utility of iPTH at 24 hours after surgery in predicting long term parathyroid function (30). iPTH of 5.8 pg/mL or lower had a poor PPV of 30% in predicting permanent hypoparathyroidism; however, a good negative predictive value (NPV) of 100%.
Measurement of postoperative PTH levels is becoming the standard in the early detection and treatment of PoSH. Early postoperative PTH and calcium based protocols are used to guide management with calcium and/or vitamin D supplementation and subsequent weaning in many centres.
Vitamin D
Given the importance of vitamin D in calcium metabolism and in particular, calcium absorption, vitamin D status has been investigated for its influence on PoSH. The role of vitamin D deficiency in postoperative hypocalcaemia is controversial, a number of studies have found that low preoperative 25-vitamin D (particularly levels lower than 25–62 nmol/L) was an independent predictor of post-thyroidectomy hypocalcaemia (15). A retrospective study evaluated the role of vitamin D deficiency in post-thyroidectomy hypocalcaemia in 213 consecutive patients who underwent total and completion thyroidectomies (31). Patients were stratified into the following vitamin D status categories: severely deficient (<25 nmol/L); deficient (<50 nmol/L); insufficient (<75 nmol/L) and sufficient (≥75 nmol/L). They found that 54% of patients in the severe deficiency group developed hypocalcaemia compared to 3.1% of those with sufficient levels.
Seasonal difference in the rates of post-thyroidectomy was studied in Montreal (32). Mascarella et al. found significant differences in the rates of hypocalcaemia between the following months: winter (8.3%); spring (7.3%); summer (1.3%); autumn (3.5%). Thyroidectomy performed in winter was significantly associated with temporary hypocalcaemia in multivariable analysis (32).
In contrast to the above studies, another retrospective study of 150 patients found no significant difference in the rates of postoperative hypocalcaemia in patients with vitamin D <50 nmol/L and those with levels ≥50 nmol/L (33). Other recent studies have also found no correlation with preoperative vitamin D levels and postoperative hypocalcaemia (34-36).
Despite this, it appears reasonable to screen for vitamin D deficiency in patients undergoing thyroid surgery as part of preoperative workup and treat them appropriately. This is not only to reduce the incidence but also severity of hypocalcaemia in addition to other long term benefits of treating vitamin D deficiency.
Magnesium
Magnesium plays an important role for secretion of PTH and release of PTH is impaired in magnesium deficiency (37).
Wilson et al. prospectively examined the association between postoperative magnesium levels and temporary hypocalcaemia in 50 patients undergoing total thyroidectomy. They found that low magnesium was an independent predictor of postoperative hypocalcaemia after adjusting variables including postoperative serum calcium. In a retrospective study of 201 patients, Garrahy et al. found that low postoperative magnesium was an independent predictor of biochemical hypocalcaemia (38). Both studies did not adjust for PTH levels. Hammerstad et al. found that patients with Graves’ who had permanent hypocalcaemia following total thyroidectomy (n=40) had a greater decline in serum magnesium (preoperatively to 48 hours post-op) (39). This finding was limited by the relatively small sample size as only four patients developed permanent hypocalcaemia in their cohort.
Cherian et al. found no association between low postoperative magnesium (<1.8 mg/dL) and postoperative hypocalcaemia in a prospective analysis of 50 patients who underwent total thyroidectomy (40). Others have also found no association between postoperative serum magnesium and post-thyroidectomy hypocalcaemia (41,42).
On this basis, routine magnesium measurements cannot be recommended, but it would be reasonable to check and treat for hypomagnesemia in patients with severe or protracted hypocalcaemia.
Alkaline phosphatase (ALP)
Sources of ALP include the bone, liver and kidney (43). Patients with primary hyperparathyroidism or hyperthyroidism may have elevated preoperative alkaline phosphate due to increase high bone turnover (44,45). These patients with high bone turnover may be at risk of postoperative hypocalcaemia due to hungry bone syndrome and this elevation could persist for up to 9 months postoperatively (44,45).
Some studies have found that patients with preoperative elevated ALP are at risk of postoperative hypocalcaemia (15). Miah et al. retrospectively studied the association between preoperative alkaline phosphate levels and postoperative hypocalcaemia in 225 patients (46). They found that preoperative ALP was significantly higher in patients who developed postoperative hypocalcaemia in univariable analysis, particularly in patients with Graves’ disease. They did not adjust for parathyroid or thyroid hormone levels.
Given the presence of other indicators of hypocalcaemia and the lack of utility of ALP levels in guiding treatment, routine measurements are not currently indicated.
Surgery related factors
Several surgical factors may help in predicting the risk of PoSH. These have been recently detailed in a systematic review (15). Factors such as surgical approach, operating technique, use of specific hemostatic techniques and parathyroid imaging are considered as preventative measures and are therefore outside the scope of this review.
Intraoperative identification
This issue is also discussed in more detail elsewhere in this journal. Large retrospective studies have shown that identification of fewer parathyroid glands at surgery may independently predict transient (16) and permanent (47) hypocalcaemia. This supports routine identification and meticulous dissection to avoid inadvertent damage and preserve long term function. In contrast, another retrospective study found higher rates of transient and permanent hypocalcaemia in patients where more parathyroid glands were identified (48). The authors used capsular dissection and avoided excessive/prolonged dissection to identify the parathyroid glands. A potential explanation for this finding could be that the glands identified represent those at or within the capsule, thus at greater risk of devascularisation. In addition, in the absence of routine search for the glands, identified glands may represent those that have been devascularised; as these are more obvious during dissection.
In a prospective study of 126 patients (49), patients who had 0–2 glands identified intraoperatively had a lower incidence of clinical hypocalcaemia compared to patients who had 3–4 glands identified (3.2% vs. 17.1%).
Puzziello et al. also evaluated the association between intraoperative identification and postoperative hypocalcaemia in a prospective multicentre study of 2,631 patients (50). Information on the surgical technique was not presented. They found that intraoperative identification of parathyroid gland is associated with increased risk of temporary hypocalcaemia, but, it significantly reduced the rates of permanent hypocalcaemia. This finding suggests that identification was associated with partial devascularisation but preservation of the gland in the long term.
In summary, the authors of this review do not advise an extensive dissection and search for parathyroid glands during surgery, but recommend that surgeons ‘look out’ for parathyroid glands and keep to capsular dissection to avoid damage to unidentified glands.
Preservation of parathyroid gland in situ
Preservation of parathyroid glands is expected to reduce the risk of PoSH. Lorente-Poch et al. proposed that this be quantified using the following formula: 4 ‒ (number of gland autotransplanted + gland in the excised specimen). The underlying assumption here is that any parathyroid gland left behind has an intact blood supply.
They examined the association between in situ preservation of parathyroid gland and postoperative hypocalcaemia in 657 patients who underwent total thyroidectomy (51). They found that fewer parathyroid glands preserved in situ significantly increased the rates of temporary and permanent post-thyroidectomy hypocalcaemia. The authors therefore argue against parathyroid autotransplantation. However, this was not a controlled study and it could be that the technique of auto-transplantation was suboptimal.
Selective parathyroid gland autotransplantation
In meta-analysis of 823 patients included in four studies who underwent bilateral thyroid surgery, selective auto-transplantation of one or more parathyroid glands was evaluated as a risk factor for PoSH (15). Studies included in the review used fairly similar techniques for autotransplantation. However, Lang et al. immediately autotransplants any devascularised gland, while Asari et al. sent a specimen for frozen section and performed the autotransplantation at the end of the procedure, during which the gland was kept in cold saline.
The meta-analysis showed an increase in the risk of temporary hypocalcaemia with autotransplantation (OR, 2.03; 95% CI, 1.44–2.86) (15). However, no association was found between autotransplantation and permanent hypocalcaemia in individual studies (15).
On this basis, it would be reasonable to advise selective autotransplantation of glands that are clearly ischaemic or have been devascularised, but assessment of this is currently subjective and may not be accurate. In the future, technologies such as fluorescent imaging of parathyroid glands may help in accurately determining tissue viability and guide selective autotransplantation.
Central neck dissection (CND)
Prophylactic central neck dissection in thyroid cancer remains controversial, particularly due to its associated morbidity. Studies have shown that prophylactic central neck dissections increase risk of temporary PoSH (14). In addition, limiting central neck dissection to ipsilateral side reduces risk of temporary PoSH compared to routine bilateral central neck dissection (14). In a meta-analysis of five observational studies with a total of 1,132 patients, Chisholm et al. compared rates of hypocalcaemia between patients who underwent total thyroidectomy with CND and those had total thyroidectomy alone (52). The CND group included patients who had either therapeutic or prophylactic CND. In addition, there was variability on the definition of temporary and permanent hypocalcaemia between studies. Patients who had total thyroidectomy with CND had an increased risk of temporary hypocalcaemia (risk difference, 7.7; 95% CI, 5.6–14.3). A total of 14 patients developed permanent hypocalcaemia; eight in prophylactic CND group and six in the total thyroidectomy alone group. There were no significant differences between groups in the rates of permanent hypocalcaemia. Another meta-analysis (15 observation studies and one RCT) compared rates of hypocalcaemia between total thyroidectomy with CND (includes prophylactic, therapeutic, bilateral and ipsilateral procedures) and total thyroidectomy alone (53). In 11 studies, total thyroidectomy with CND significantly increased risk of temporary hypocalcaemia (262/845, 31% vs. 239/1,478, 16%). No significant difference was seen in the rates of permanent hypocalcaemia between the two groups. In addition, there was no significant difference between total thyroidectomy (TT) alone and TT with CND in locoregional recurrence rates. In another meta-analysis, Lang et al. compared surgical morbidity and short-term local regional recurrence between patients who had TT with prophylactic CND and those who had TT alone (54). Pooled results of 11 studies that examined temporary hypocalcaemia found that TT with prophylactic CND had significantly higher rates [336/1,294 (26.0%)] vs. TT alone [114/1,330 (10.8%)]. Again, no significant difference was seen in the rates of long term hypocalcaemia (2% vs. 1.2%). However, total thyroidectomy with CND significantly reduced the risk of locoregional recurrence compared to total thyroidectomy alone (4.7% vs. 8.6%).
Although therapeutic CND is not controversial, the practice of prophylactic CND is variable across centres. Local rates of PoSH following CND should therefore be used to guide surgeons and patients in deciding on whether prophylactic CND should be done.
Inadvertent excision of parathyroid gland
This factor is discussed in more detail elsewhere in this journal. Rates of inadvertent parathyroidectomy range from 3.7% to 21.6% (55). This is higher in patients who had concomitant central neck dissection (56). In most instances, only one gland is excised, although occasionally two or more parathyroid glands may be removed (55). Of these, the rates of intrathyroidal position ranges from 5.2–68.8% (57). A meta-analysis of 1,482 patients involved in four studies demonstrated that inadvertent excision was significantly associated with transient hypocalcaemia (OR, 1.90; 95% CI, 1.31–2.74) (15).
Although intra-thyroidal parathyroid excision cannot be avoided, the surgeon should take care to preserve extra-thyroidal glands by ensuring that the dissection is at the capsular plane at all times. Inadvertent excision of extra-thyroidal glands could be considered a surrogate marker for lack of capsular dissection and therefore a predictor for PoSH.
Surgical volume
High volume surgeons may represent surgeons with endocrine or thyroid specialisation. In a prospective study, Gonzalez-Sanchez et al. examined the association between surgical volume (surgeons preforming <5 vs. >40 cases per year) and postoperative hypocalcaemia (58). The low volume group had a significantly higher rate of hypocalcaemia compared to high volume surgeons at 6 months (8/16 vs. 8/130) and 12 months (3/16 vs. 3/130) postoperatively. They concluded that specialised training in endocrine surgery may account for differences seen in complication rates. No recommendation was given on the minimal number of cases required to achieve optimal results or to be considered a high volume surgeon. In another multicentre study however, Thomusch et al. found no link between surgical volume (<10 and 10–50 compared to >50 cases per year) and temporary or permanent postsurgical hypocalcaemia (47). However, other clinical outcomes also improve with the volume of thyroid surgery performed and most national and international guidelines now advocate that thyroid surgery be avoided by low or very low volume surgeons.
Other surgical related factors identified as potential predictors of temporary hypocalcaemia in multivariable analysis in various studies are longer duration of surgery (59), reoperation for bleeding (16,59), and re-operative surgery for recurrent goitre (60).
Patient and disease related factors
Age at surgery
Association between advanced age and PoSH could be due to vitamin D deficiency with advance age (61). There are conflicting reports with regards to age and the developments of postoperative hypocalcaemia. Some studies have identified older age as risk factors while others have found younger aged to be at risk (15). In a meta-analysis of 2,576 patients (from five studies) who underwent bilateral surgery, age at surgery was not significantly associated with postoperative hypocalcaemia (15).
Female gender
Female gender has been identified as an independent risk factor for the development of post-thyroidectomy hypocalcaemia (16,47,62). In addition, a meta-analysis of 3,860 patients (from 10 studies) who underwent bilateral thyroid surgery found that female had a significantly higher rates of temporary hypocalcaemia compared to male patients (OR, 1.7; 95% CI, 1.03–2.8). The mechanism of this phenomenon however remains unclear despite some speculation on the ‘smaller operative field’ in females (61) and the prevalence of vitamin D deficiency, in female patients with Graves’ disease (63).
Graves’ disease
Hungry bone syndrome is an accepted mechanism for the link between Graves’ disease and temporary hypocalcaemia. In addition, parathyroid preservation during surgery for Graves’ disease may be more technical challenging given the increased gland vascularity.
The association between Graves’ disease and postoperative hypocalcaemia has been studied in a meta-analysis. The meta-analysis included six studies with a total number of 6,779 patients who underwent bilateral thyroid surgery (15). Graves’ disease significantly increased the risk of postoperative hypocalcaemia (OR, 1.8; 95% CI, 1.4–2.4). Furthermore, Thomusch et al. found that Graves’ disease (when compared to multinodular goitre) was an independent predictor of permanent hypocalcaemia (47).
In patients with Graves’ disease, adequate optimisation of thyroid status, treatment of existing vitamin D deficiency, reduction of vascularity with preoperative Lugol’s iodine and careful capsular dissection should help reduce PoSH rates.
Weight of excised specimen
Heavier specimen may represent more extensive surgery, thus predisposing to PoSH. Heavier weight of resected specimen had been shown to be an independent predictor of temporary (64) and six month hypocalcaemia (59).
Retrosternal goitre
Surgery for retrosternal goitre could predispose to PoSH due to extent of surgery and longer dissection. In a multicentre study of 19,662, Testini et al. examined the morbidity of retrosternal goitres (65). Patients who had surgery (cervical approach or sternotomy) for retrosternal goitre had significantly higher rates of temporary and permanent hypocalcaemia compared to cervical goitre group (temporary, 33.8% vs. 21.6%; permanent, 2.2% vs. 1.0%).
Care should therefore be taken with large multinodular and retrosternal goitres to help reduce this risk. In patients with predominantly unilateral disease, a hemithyroidectomy should therefore be considered.
Conclusions
This review summarises literature on the epidemiology of PoSH. Given the increasing numbers of thyroid surgery worldwide, PoSH is assuming increasing significance and adding to the burden of disease in the community. The epidemiological data are hindered by the lack of a widely accepted consensus on the definition of both temporary and long term or permanent hypocalcaemia and/or hypoparathyroidism. It is imperative that medical and surgical societies and associations arrive at a consensus on how to define this condition to enable appropriate treatment, comparison of studies in literature and effective assessment of preventative measures.
Risk factors for the occurrence of PoSH may be classified as modifiable or treatable (such as Vitamin D deficiency or hypomagnesemia) or non-modifiable (such as postoperative PTH level or Graves’ disease). A more accurate understanding of risk attributable to individual risk factors would help in a better prediction of risk of PoSH and this can be factored into the discussions regarding the risks and benefits of surgery. When the risk is considered high, it may be possible and appropriate to modify approach or extent of surgery in ‘high risk’ patients or use some of the novel preventative measures such as fluorescent imaging for parathyroid identification or preservation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sitges-Serra A, Ruiz S, Girvent M, et al. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687-95. [Crossref] [PubMed]
- Mandel SJ, Larsen PR, Davies TF. Thyrotoxicosis. Williams textbook of endocrinology. 12 ed. Philadephia: Elsevier saunders, 2011:362-405.
- Mehta N, Watts NB, Welge JA, et al. Comparison of serum calcium change following thyroid and nonthyroid neck surgery. Otolaryngol Head Neck Surg 2006;134:901-6. [Crossref] [PubMed]
- Watson CG, Steed DL, Robinson AG, et al. The role of calcitonin and parathyroid hormone in the pathogenesis of post-thyroidectomy hypocalcemia. Metabolism 1981;30:588-9. [Crossref] [PubMed]
- Hannan FM, Thakker RV. Investigating hypocalcaemia. Bmj 2013;346:f2213. [Crossref] [PubMed]
- Underbjerg L, Sikjaer T, Mosekilde L, et al. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res 2014;29:2504-10. [Crossref] [PubMed]
- Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2010;32:279-83. [PubMed]
- Chadwick D. The British association of endocrine and thyroid surgeons, fifth national audit report. Oxfordshire: Dendrite Clinical Systems, 2017.
- Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol 2015;173:G1-20. [Crossref] [PubMed]
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: postoperative hypoparathyroidism--definitions and management. Endocr Pract 2015;21:674-85. [Crossref] [PubMed]
- Lang BH, Chan DT, Chow FC, et al. The association of discolored parathyroid glands and hypoparathyroidism following total thyroidectomy. World J Surg 2016;40:1611-7. [Crossref] [PubMed]
- Mittendorf EA, Merlino JI, McHenry CR. Post-parathyroidectomy hypocalcemia: incidence, risk factors, and management. Am Surg 2004;70:114-9; discussion 119-20. [PubMed]
- Negm H, Mosleh M, Fathy H, et al. Thyroid and parathyroid dysfunction after total laryngectomy in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol 2016;273:3237-41. [Crossref] [PubMed]
- Antakia R, Edafe O, Uttley L, et al. Effectiveness of preventative and other surgical measures on hypocalcemia following bilateral thyroid surgery: a systematic review and meta-analysis. Thyroid 2015;25:95-106. [Crossref] [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Pisaniello D, Parmeggiani D, Piatto A, et al. Which therapy to prevent post-thyroidectomy hypocalcemia? G Chir 2005;26:357-61. [PubMed]
- Underbjerg L, Sikjaer T, Mosekilde L, et al. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res 2013;28:2277-85. [Crossref] [PubMed]
- Powers J, Joy K, Ruscio A, et al. Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res 2013;28:2570-6. [Crossref] [PubMed]
- Moonesinghe R, Chang MH, Truman BI. Health insurance coverage - United States, 2008 and 2010. MMWR Suppl 2013;62:61-4.
- Astor MC, Lovas K, Debowska A, et al. Epidemiology and health-related quality of life in hypoparathyroidism in Norway. J Clin Endocrinol Metab 2016;101:3045-53. [Crossref] [PubMed]
- Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope 2013;123:2056-63. [Crossref] [PubMed]
- Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer 2016;23:313-22. [Crossref] [PubMed]
- Nouraei SA, Virk JS, Middleton SE, et al. A national analysis of trends, outcomes and volume-outcome relationships in thyroid surgery. Clin Otolaryngol 2017;42:354-65. [Crossref] [PubMed]
- Wiltshire JJ, Drake TM, Uttley L, et al. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid 2016;26:1541-52. [Crossref] [PubMed]
- Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 1998;22:718-24. [Crossref] [PubMed]
- Pisanu A, Cois A, Piu S, et al. Factors predicting outcome of hypocalcaemia following total thyroidectomy. Chir Ital 2003;55:35-40. [PubMed]
- Lecerf P, Orry D, Perrodeau E, et al. Parathyroid hormone decline 4 hours after total thyroidectomy accurately predicts hypocalcemia. Surgery 2012;152:863-8. [Crossref] [PubMed]
- Schlottmann F, Arbulú AL, Sadava EE, et al. Algorithm for early discharge after total thyroidectomy using PTH to predict hypocalcemia: prospective study. Langenbecks Arch Surg 2015;400:831-6. [Crossref] [PubMed]
- Julian MT, Balibrea JM, Granada ML, et al. Intact parathyroid hormone measurement at 24 hours after thyroid surgery as predictor of parathyroid function at long term. Am J Surg 2013;206:783-9. [Crossref] [PubMed]
- Al-Khatib T, Althubaiti AM, Althubaiti A, et al. Severe vitamin D deficiency: a significant predictor of early hypocalcemia after total thyroidectomy. Otolaryngol Head Neck Surg 2015;152:424-31. [Crossref] [PubMed]
- Mascarella MA, Forest VI, Nhan C, et al. Seasonal difference in postthyroidectomy hypocalcemia: a montreal-based study. Otolaryngol Head Neck Surg 2016;154:263-7. [Crossref] [PubMed]
- Cherian AJ, Ponraj S, Gowri SM, et al. The role of vitamin D in post-thyroidectomy hypocalcemia: still an enigma. Surgery 2016;159:532-8. [Crossref] [PubMed]
- Griffin TP, Murphy MS, Sheahan P. Vitamin D and risk of postoperative hypocalcemia after total thyroidectomy. JAMA Otolaryngol Head Neck Surg 2014;140:346-51. [Crossref] [PubMed]
- Lee GH, Ku YH, Kim HI, et al. Vitamin D level is not a predictor of hypocalcemia after total thyroidectomy. Langenbecks Arch Surg 2015;400:617-22. [Crossref] [PubMed]
- Lang BH, Wong KP, Cheung CY, et al. Does preoperative 25-hydroxyvitamin D status significantly affect the calcium kinetics after total thyroidectomy? World J Surg 2013;37:1592-8. [Crossref] [PubMed]
- Anast CS, Winnacker JL, Forte LR, et al. Impaired release of parathyroid hormone in magnesium deficiency. J Clin Endocrinol Metab 1976;42:707-17. [Crossref] [PubMed]
- Garrahy A, Murphy MS, Sheahan P. Impact of postoperative magnesium levels on early hypocalcemia and permanent hypoparathyroidism after thyroidectomy. Head Neck 2016;38:613-9. [Crossref] [PubMed]
- Hammerstad SS, Norheim I, Paulsen T, et al. Excessive decrease in serum magnesium after total thyroidectomy for Graves' disease is related to development of permanent hypocalcemia. World J Surg 2013;37:369-75. [Crossref] [PubMed]
- Cherian AJ, Gowri M, Ramakant P, et al. The role of magnesium in post-thyroidectomy hypocalcemia. World J Surg 2016;40:881-8. [Crossref] [PubMed]
- Cavicchi O, Piccin O, Caliceti U, et al. Accuracy of PTH assay and corrected calcium in early prediction of hypoparathyroidism after thyroid surgery. Otolaryngol Head Neck Surg 2008;138:594-600. [Crossref] [PubMed]
- Wu SD, Gao L. Is routine calcium supplementation necessary in patients undergoing total thyroidectomy plus neck dissection? Surg Today 2011;41:183-8. [Crossref] [PubMed]
- Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014;29:269-78. [Crossref] [PubMed]
- Dhanwal DK. Thyroid disorders and bone mineral metabolism. Indian J Endocrinol Metab 2011;15:S107-12. [Crossref] [PubMed]
- Witteveen JE, van Thiel S, Romijn JA, et al. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol 2013;168:R45-53. [Crossref] [PubMed]
- Miah MS, Mahendran S, Mak C, et al. Pre-operative serum alkaline phosphatase as a predictive indicator of post-operative hypocalcaemia in patients undergoing total thyroidectomy. J Laryngol Otol 2015;129:1128-32. [Crossref] [PubMed]
- Thomusch O, Machens A, Sekulla C, et al. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003;133:180-5. [Crossref] [PubMed]
- Prazenica P, O'Keeffe L, Holy R. Dissection and identification of parathyroid glands during thyroidectomy: association with hypocalcemia. Head Neck 2015;37:393-9. [Crossref] [PubMed]
- Sheahan P, Mehanna R, Basheeth N, et al. Is systematic identification of all four parathyroid glands necessary during total thyroidectomy?: a prospective study. Laryngoscope 2013;123:2324-8. [Crossref] [PubMed]
- Puzziello A, Rosato L, Innaro N, et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine 2014;47:537-42. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359-67. [Crossref] [PubMed]
- Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope 2009;119:1135-9. [Crossref] [PubMed]
- Shan CX, Zhang W, Jiang DZ, et al. Routine central neck dissection in differentiated thyroid carcinoma: a systematic review and meta-analysis. Laryngoscope 2012;122:797-804. [Crossref] [PubMed]
- Lang BH, Ng SH, Lau LL, et al. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid 2013;23:1087-98. [Crossref] [PubMed]
- Zhou HY, He JC, McHenry CR. Inadvertent parathyroidectomy: incidence, risk factors, and outcomes. J Surg Res 2016;205:70-5. [Crossref] [PubMed]
- Sitges-Serra A, Gallego-Otaegui L, Suárez S, et al. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery 2017;161:712-9. [Crossref] [PubMed]
- Manatakis DK, Balalis D, Soulou VN, et al. Incidental parathyroidectomy during total thyroidectomy: risk factors and consequences. Int J Endocrinol 2016;2016:7825305. [PubMed]
- González-Sánchez C, Franch-Arcas G, Gómez-Alonso A. Morbidity following thyroid surgery: does surgeon volume matter? Langenbecks Arch Surg 2013;398:419-22. [Crossref] [PubMed]
- Hallgrimsson P, Nordenstrom E, Almquist M, et al. Risk factors for medically treated hypocalcemia after surgery for Graves' disease: a Swedish multicenter study of 1,157 patients. World J Surg 2012;36:1933-42. [Crossref] [PubMed]
- Cappellani A, Di Vita M, Zanghi A, et al. The recurrent goiter: prevention and management. Ann Ital Chir 2008;79:247-53. [PubMed]
- Erbil Y, Barbaros U, Temel B, et al. The impact of age, vitamin D(3) level, and incidental parathyroidectomy on. Am J Surg 2009;197:439-46. [Crossref] [PubMed]
- Bove A, Bongarzoni G, Dragani G, et al. Should female patients undergoing parathyroid-sparing total thyroidectomy receive routine prophylaxis for transient hypocalcemia? Am Surg 2004;70:533-6. [PubMed]
- Yamashita H, Noguchi S, Murakami T, et al. Calcium and its regulating hormones in patients with graves disease: sex differences and relation to postoperative tetany. Eur J Surg 2000;166:924-8. [Crossref] [PubMed]
- Yamashita H, Noguchi S, Tahara K, et al. Postoperative tetany in patients with Graves' disease: a risk factor analysis. Clin Endocrinol (Oxf) 1997;47:71-7. [Crossref] [PubMed]
- Testini M, Gurrado A, Avenia N, et al. Does mediastinal extension of the goiter increase morbidity of total thyroidectomy? A multicenter study of 19,662 patients. Ann Surg Oncol 2011;18:2251-9. [Crossref] [PubMed]


