The effect of long-term thyroid-stimulating hormone suppressive therapy on the gonadal steroid hormones of patients with thyroid carcinoma after surgery
Introduction
Follicular cell-derived thyroid cancer is the most frequent endocrine cancer, with a rapidly rising incidence in the recent years. This carcinoma can be classified into several histological types, including papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and anaplastic thyroid cancer (ATC). PTC and FTC are differentiated thyroid carcinoma (DTC), while ATC is deadly undifferentiated thyroid cancer. PTC, accounting for 85–90%, is the most common one among all the thyroid malignancies. Treatment of thyroid cancer includes surgery, radioactive iodine (I131) and thyroid-stimulating hormone (TSH) suppressive therapy.
TSH-suppressive therapy was firstly applied by Dr. Dunhill in 1973. Gradually, it developed into a special therapy with more routinization, rationalization and individualization. Its curative effect has been approved broadly (1). It is recommended by guidelines that patients with DTC should conduct the TSH-suppressive therapy using exogenous levothyroxine after initial surgical treatment (2). The exogenous levothyroxine decreases the endogenic level of TSH to normal value. It does not only supplement the lack of thyroid hormones after surgery, but also inhibits the risk of recurrence and metastasis (3-5). However, with the high survival rate after the initial treatment of DTC, there is growing interest in the potential harmful effects brought on by the long-term TSH-suppressive therapy. In particular, increased cardiovascular and fracture risk by exogenous subclinical thyrotoxicosis have been reported (6-11). Therefore, not only the potential benefit but also the potential risk of TSH suppression should be considered. This study analyzed the effect of long-term TSH suppression therapy on gonadal steroid hormones by postoperative follow-up, aiming to explore the potential relationship and further to direct the clinical application.
Methods
Patients
From 2008 to 2011, totally 238 patients were recruited in this study, who underwent thyroid surgery and subsequent TSH suppression treatment in Department of thyroid Surgery, China-Japan Union Hospital, Jilin University. All the patients agreed to participate into this study. Patients with surgical menopause were ruled out. Then their postoperative follow-up data (3–8 years) were collected, including operational method, pathological diagnosis, whether processed radioiodine therapy and the period/dose of TSH suppression treatment. In addition, the menstrual cycle, menstruation quantity, whether accompanied with dysmenorrheal and menstrual disorder or not, date of last menstrual period, ages of menopause and so on were also collected.
Biochemical tests
Blood was collected on the day when surgery was performed. Blood samples were drawn in the morning after overnight fasting, immediately processed to obtain serum. Measurements were performed for the following parameters: follicle-stimulating hormone (FSH) reference range: male adult (0.95–11.95 IU/L), female follicular phase (3.03–8.08 IU/L), ovulatory period (2.55–16.69 IU/L), luteal phase (1.38–5.47 IU/L), menopause (26.72–133.41 IU/L); testosterone (T) reference range: male (4.94–32.01 nmol/L), female (0.38–1.97 nmol/L); estradiol (E2) reference range: male adult (40.40–161.50 pmol//L), female follicular phase (77.10–921.20 pmol//L), ovulatory period (139.50–2,381.80 pmol//L), luteal phase (77.1–1,145.00 pmol//L), menopause (1.00–102.80 pmol//L); TSH (reference range: 0.37–4.94 mIU/L), free T3 (FT3, reference range: 3.10–6.80 pmol/L); free T4 (FT4, reference range: 12.0–22.0 pmol/L).
Grouping
According to the level of TSH, patients were divided into three groups: TSH <0.1 IU/L, 0.1 IU/L ≤ TSH <0.5 IU/L and TSH ≥0.5 IU/L, while according to the period of suppression therapy, patients were classified into two parts: 3–5 and 6–8 years. In addition, all the female patients were further divided into sexual maturity (follicular phase, ovulatory period, and luteal phase), menopausal transition and postmenopausal period. Menopausal women include preoperative menopause and postoperative menopause.
Statistical analysis
All statistical analyses were performed with SPSS17.0 software. Values with normal distribution were expressed as mean ± SD, while values with non-normal distribution were expressed as median (interquartile range). Comparison between two or multiple groups of continuous variables was performed by Wilcoxon-Mann-Whitney test or ANOVA analysis. Comparison of two groups of categorical variables was performed using the Pearson χ2 test or Fisher’s exact test. Data with value of P<0.05 were considered to be statistically significant.
Results
Subject characteristics
A total of 238 patients were recruited in this study, including 51 male patients (mean age 47.20±8.69 years) and 187 female patients (mean age 46.38±8.60 years). The ratio of male to female was 1:3.67.
The effect of TSH suppression therapy on hormone level in male patients
According to the level of TSH, patients were divided into three groups: TSH <0.1 IU/L, 0.1 IU/L ≤ TSH <0.5 IU/L and TSH ≥0.5 IU/L, while according to the period of suppression therapy, patients were classified into two parts: 3–5 and 6–8 years. There were 20 male patients taking lower level (TSH<0.1 IU/L) of TSH, and 13 out of 20 patients took TSH suppression therapy for 3–5 years, while 7 out of 20 patients continued the therapy for around 6–8 years. Similarly, there were 15 patients taking medium level (0.1 U/L ≤ TSH <0.5 U/L) of TSH treatment (11 patients for 3–5 years and 4 patients for 6–8 years, respectively) while 16 patients taking higher level (TSH ≥0.5 U/L) of TSH treatment (11 patients for 3–5 years and 5 patients for 6–8 years, respectively).
The long-term effect of TSH suppression therapy was studied by comparing subgroups of patients with different levels of TSH and patients with different durations of TSH suppression therapy. As illustrated in Table 1, on the one hand, among all the patients taking TSH treatment for 3–5 years, the level of T was 17.75±8.59 vs. 13.91±6.01 vs. 12.67±3.91 nmol/L in patients who conducted TSH for low, medium and high level, respectively. On the other hand, for all the patients taking TSH treatment for 6–8 years, the level of T was 18.21±6.69 vs. 14.98±10.37 vs. 11.88±3.33 nmol/L respectively. No significant statistic difference was observed among patients in terms of different levels of TSH treatment (P=0.159 and 0.330 respectively). From another point of view, the group of patients who persisted TSH treatment for 3–5 years did not differ from patients for 6–8 years no matter the level of TSH was low, medium or high (P=0.902, 0.805 and 0.701). In brief, neither the level nor the duration of TSH treatment had any effect on T in male patients.
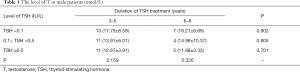
Full table
In addition, the change of E2 in male patients was also detected and compared in the same manner. As shown in Table 2, with low level of TSH treatment (TSH <0.1 IU/L), the level of E2 in patients who continued TSH treatment for 3–5 years was 103.85±23.29 pmol/L while in patients who continued for 6–8 years the level of E2 was 117.14±21.27 pmol/L. There was no statistic difference between the above subgroups as the P was 0.226 which was greater than 0.05. As for the medium level of TSH treatment (0.1 U/L ≤ TSH <0.5 U/L), the level of E2 did not differ by the duration of TSH treatment (114.64±36.65 pmol/L in 3–5 years vs. 106.00±26.82 pmol/L in 6–8 years, P=0.676). Same phenomenon was observed in patients with high dose (TSH ≥0.5 U/L) of TSH treatment (103.64±17.55 pmol/L in 3–5 years vs. 108.00±10.70 pmol/L in 6–8 years, P=0.619). From another perspective, the effect of different levels of TSH treatment on E2 in male patients was analyzed in two durations of TSH treatment: 3–5 and 6–8 years. Neither of them showed difference among various doses of TSH. Taken together, neither the level nor the duration of TSH treatment had any function on E2 in male patients.
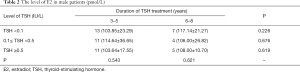
Full table
The effect of TSH suppression therapy on hormone level in female patients
Because the female hormone level changed according to different phases, in order to compare the discrepancy accurately, all the female patients were divided into several phases, such as follicular phase, luteal phase, menopause and post-menopausal period. The female patients distributed as listed in Table 3.
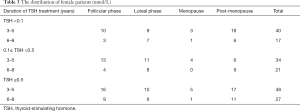
Full table
First of all, the effects of TSH therapy on T level in female patients were clarified. As presented in Table 4, in the subgroup of patients with TSH treatment for 3–5 years, patients who took high dose of TSH (TSH ≥0.5 U/L) obtained the lower T level compared with the group of medium dose (1.08±0.34 vs. 1.36±0.46 nmol/L, P=0.001). It was also lower than the low dose group, although not statistically significant (1.08±0.34 vs. 1.2±0.36 nmol/L, P=0.079). On the contrary, the T level did not change in terms of the different levels of TSH in patients with TSH treatment for 6–8 years (1.17±0.51 vs. 1.34±0.35 vs. 1.19±0.52 nmol/L, P=0.426). What’s more, the duration of TSH treatment did not have special effect on the T level under the same dose of treatment (P=0.596, 0.877, and 0.292 respectively). Above results demonstrated that the high level of TSH together with duration for less than 5 years would result in lower T level in female patients.

Full table
Secondly, to address possible effect of TSH-suppressive therapy on E2 level in female patients, we performed further comparison in follicular phase, luteal phase and post-menopausal period dividedly. According to Table 5, under the low level (TSH <0.1 IU/L) of TSH treatment, patients either in follicular phase or in luteal phase presented a similar E2 level no matter how long did they continue TSH treatment (181.5 vs. 145.0 pmol/L, P=0.866 in follicular phase; 319.0 vs. 291.0 pmol/L, P=0.958 in luteal phase). Similar observation was also obtained in high dose (TSH ≥0.5 U/L) of TSH treatment (213.5 vs. 154.0 pmol/L, P=0.157 in follicular phase; 240.0 vs. 275.0 pmol/L, P=0.828 in luteal phase). However, in the medium dose of TSH treatment, patients in luteal phase showed significant difference between two separate durations as the E2 level was 196.0 pmol/L in 3–5 years group vs. 442.5 pmol/L in 6–8 years group, P=0.018, which meant that the longer TSH treatment would lead to higher level of E2. Meanwhile, either the level or the duration of TSH treatment changed the E2 level in postmenopausal female patients (Table 6).

Full table

Full table
Thirdly, the level of FSH were also detected and analyzed by the similar method (Tables 7,8). Unfortunately, the level of FSH did not show any change in terms of the dose and the duration of TSH treatment.

Full table

Full table
In conclusion, among the three important female hormone, long-term of TSH suppression therapy had special function on T and E2 by different ways but not FSH.
The effect of TSH suppression therapy on menstruation and menopause
When being ovarian disability, it will lead to failure of ovulation, decline of estrogen levels and irregular menstruation. As clarified above, long-term of TSH treatment lead to decrease of hormone level. So we wonder to further explore whether this treatment would affect female menstruation and menopause.
On the one hand, the menstruation condition was reflected in three aspects: menstrual volume (decrease, normal and increase), dysmenorrhea (yes or no) and menstrual cycle (irregular or regular). As listed in Table 9, all three doses of TSH treatment showed similar effect on menstruation condition. The menstrual volume of majority female patients was normal (accounting for around 66%), but no difference within three subgroups of individual doses (P=0.764). Meanwhile, most of them did not have dysmenorrhea and their menstrual cycles were almost regular. What is important, neither the patients without dysmenorrhea nor the patients with regular menstrual cycle differ by the dose of TSH treatment (P=0.087 and P=0.186).
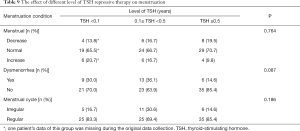
Full table
In similarity, menstrual volume, dysmenorrhea condition and menstrual cycle did not show any difference in terms of duration of TSH treatment (P=0.701, 0.412 and 0.507 respectively) (Table 10). All above suggested that the menstruation were not affected by the long-term TSH treatment.
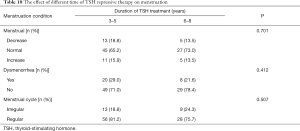
Full table
On another hand, according to the time when female patients become menopause, all the menopausal patients were grouped into two parts: postoperative menopause and preoperative menopause. Then the therapy related characters were analyzed (Table 11). First of all, neither menopause age nor the level of hormone including (E2, T, and FSH) presented any difference between postoperative menopause and preoperative menopause. Secondly, no significant discrepancy were obtained in terms of operative approach (total resection, subtotal resection), whether conducted I131 radionuclide therapy and long-term of TSH treatment. Finally, the cancer risk stratification were also taken into consideration, however, any kind of risk level did not show significant function on menopause. Of note, we did not find significant effect of long term TSH therapy on female menopause.
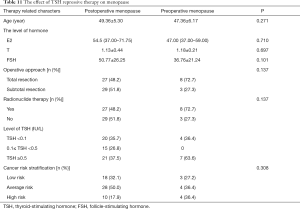
Full table
Discussion
The purpose of our study was to assess the effect of long term TSH-suppressive therapy on hormone level in patients operated for DTC. According to the protocol of initial, post initial and long-term management of these patients, thyroid surgery, radionuclide therapy and TSH-suppressive therapy are used to prevent disease progression or recurrence. Patients with DTC are highly recommended to conduct the TSH-suppressive therapy after surgical treatment (2), which supplying the lack of thyroid hormones after surgery and inhibiting the risk of recurrence and metastasis (3-5). However, the evaluation of possible adverse effects of the therapy is essential, and hormone level change is one of the concerns.
As we know, estrogen consists of E2, estrone and estriol. Among of them, E2 shows the strongest activity, so it is usually detected to reflect the ability of ovary. In man, however, one third of estrogen in man comes from testis while the other two third of estrogen is transformed from androgen outside of testis. Our attention was paid to the effect of TSH repressive therapy on estrogen. In this study, the different doses and durations of TSH did not play any role on E2 in male. This is consistent with another investigation, which reported TSH repressive therapy showed just little effect on bone mineral density in male DTC patients (12). On the contrary, Sandra et al. (13) found that long-term of high doses T4 would lead to the retrogression of pituitary and ovarian functional cell, which further decreased the level of luteinizing hormone (LH) and E2 in rat. In addition, there were special relationships between the severity and drug doses. Our data are consistent with those studies, which found that the E2 level was significant lower in the beginning (less than 5 years) of TSH treatment than long-term (more than 5 years) of TSH treatment, which might due to the strict control of TSH dose in the very beginning. When developing into menopause phase the disability of ovary result in the stop of E2 secretion. That could explain no function of TSH treatment on E2 was obtained in female patients who were under menopause in this study. Similar results were obtained in the study (14), which showed that when being lack of E2, thyroid hormones played little role on bone metabolism. So we speculated that the drug-induced subclinical hyperthyroidism affected E2 level by the targets of gonad but not the transform of non-hormone. What is more, the function of thyroid hormones on E2 will decline when gonad organ degenerated.
T is the principal male sex hormone, secreted primarily by the testes and, to a lesser extent, by adrenal glands. This hormone’s androgenic effects are responsible for the maturation of male sexual organs, as well as for secondary sexual characteristics (growth of beard, axillary, and pubic hair, and deepening of voice). T is needed for the development of normal sperm production and contributes to sex drive. It also has anabolic effects, including promotion of muscle mass, strength, bone density, and maturation. Some scholars believe that TSH played an important role on bone metabolism, the activity of osteoblast and osteoclast increased significantly in hyperthyroidism or subclinical hyperthyroidism condition, and the effect of osteoclasts were much more obvious (15). Some others demonstrated the decline of T made bone metabolism in a negative balance, which was one of important reasons for male osteoporosis. Blouin’s (16) investigation found that resection of testis reduced the concentration of T in blood, and further resulted in decrease of bone mineral density. Consistently, it was explored by D’Amore (17) that T replacement therapy could improve symptom in male osteoporosis patients. In our study, no difference was found in T level among either different doses of TSH or two durations of TSH treatment. Therefore, we assumed that the targets of TSH repressive therapy on T level were not leydig cells so that the function was not obvious. In addition, it was of high possibility that TSH playing a direct role on bone cell metabolism, but not drug-induced reduction of T levels, lead to male osteoporosis. T is also produced in small quantities in the ovaries but the level of T is much lower in women than in man. T levels decrease with age, which is associated with increase of atherosclerosis and cardiovascular risk. Our results illustrated that the high dose of TSH treatment taking for less than 5 years resulted in a lower level of T. But the potential reasons need further investigation.
FSH was selected in our research to predict the ovary ability. As clarified in results part, neither the dose nor the duration of TSH treatment presented any function on FSH level, which might because that as pituitary secreted hormone, FSH could not affected obviously by high dose of TSH. When being menopause, the dysfunction of ovary and the decrease of E2 secretion gave rise to the level of FSH and LH by negative feedback. However, our results showed that with the duration of TSH treatment increasing, the level of FSH presented a decline but without statistically significant.
Finally, we focus on the role of TSH treatment on female menstruation and menopause. No obvious effect of long term TSH treatment was observed on menstrual volume, dysmenorrhea and menstrual cycle. Meanwhile, the TSH repressive treatment did not show any function on female menopause. Because the sample of patients being menopausal transition period was too small, the effect of TSH treatment on menopausal transition was hard to obtain. However, a study provided convincing evidence to state that the abnormal TSH level did not affect menopausal transition related symptom (18).
From the whole view of all above illustration, there were still several limitations as follows: first of all, the standard control group in present study should be patients without TSH treatment after initial surgery, but it was difficult to obtain because of the disease specificity. So we have to compare the difference between different doses and durations of TSH treatment. Secondly, as a cross-sectional descriptive study, this study was lack of the comparison of hormone level between preoperative and postoperative. It is necessary to design prospective animal experiment to obtain much stronger convincing evidence. Lastly, the time of examination was not arranged to be the same period of menstrual cycle; all related information came from patients themselves, which might lead to the staging error.
Conclusions
In summary, the long term of TSH repressive therapy after surgery did not affect T and E2 level in male patients. As for female patients, the impact was mainly reflected in the T and E2 levels especially in female sexual maturity but not FSH level. In addition, TSH treatment did not play any role on menstruation or menopause. However, the specific mechanism need to be further explored. According to 2015 ATA treatment guidelines, it is highly recommended that in order to promote the rationalization and individualization of TSH suppression therapy, it is necessary to minimize the adverse effects without affecting the therapeutic effect. With regard to patients who need TSH suppression therapy, particularly female patients and elderly patients, it is recommended to detect sex hormones before and after surgery, and assess reproductive function, bone density and cardiac function when necessary.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board of China-Japan Union Hospital of Jilin University [2008] and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008) and written informed consent was obtained from all patients.
References
- Hurley JR. Historical note: TSH suppression for thyroid cancer. Thyroid 2011;21:1175-6. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167-9. [Crossref] [PubMed]
- Crile G Jr, Antunez AR, Esselstyn CB Jr, et al. The advantages of subtotal thyroidectomy and suppression of TSH in the primary treatment of papillary carcinoma of the thyroid. Cancer 1985;55:2691-7. [Crossref] [PubMed]
- Crile G Jr. A second opinion about thyroid hormone treatment. Surgery 1988;103:268-9. [PubMed]
- Schlote B, Nowotny B, Schaaf L, et al. Subclinical hyperthyroidism: physical and mental state of patients. Eur Arch Psychiatry Clin Neurosci 1992;241:357-64. [Crossref] [PubMed]
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012;379:1142-54. [Crossref] [PubMed]
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008;29:76-131. [Crossref] [PubMed]
- Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid 2010;20:135-46. [Crossref] [PubMed]
- Al-Abadi AC. Subclinical thyrotoxicosis. Postgrad Med J 2001;77:29-32. [Crossref] [PubMed]
- Parle JV, Maisonneuve P, Sheppard MC, et al. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001;358:861-5. [Crossref] [PubMed]
- Mendonça Monteiro de Barros G, Madeira M, Vieira Neto L, et al. Bone mineral density and bone microarchitecture after long-term suppressive levothyroxine treatment of differentiated thyroid carcinoma in young adult patients. J Bone Miner Metab 2016;34:417-21. [Crossref] [PubMed]
- Cecconi S, Rossi G, Coticchio G, et al. Influence of thyroid hormone on mouse preantral follicle development in vitro. Fertility Sterility 2004;81 Suppl 1:919-24. [Crossref] [PubMed]
- Simsek G, Uzun H, Karter Y, et al. Effects of osteoporotic cytokines in ovary-intact and ovariectomised rats with induced hyperthyroidism; is skeletal responsiveness to thyroid hormone altered in estrogen deficiency? Tohoku J Exp Med 2003;201:81-9. [Crossref] [PubMed]
- Pollock MA, Jones A. Laboratory based study of undetectable thyroid stimulating hormone. J Clin Pathol 1989;42:1237-40. [Crossref] [PubMed]
- Blouin S, Gallois Y, Moreau MF, et al. Disuse and orchidectomy have additional effects on bone loss in the aged male rat. Osteoporos Int 2007;18:85-92. [Crossref] [PubMed]
- D’Amore M, Bottalico C, D’Amore S, et al. Sex hormones and male osteoporosis. A physiologic prospective for prevention and therapy. Minerva Med 2000;91:283-9. [PubMed]
- Sowers M, Luborsky J, Perdue C, et al. Thyroid stimulating hormone (TSH) concentrations and menopausal status in women at the mid-life: SWAN. Clin Endocrinol (Oxf) 2003;58:340-7. [Crossref] [PubMed]


