The use of ICG enhanced fluorescence for the evaluation of parathyroid gland preservation
Introduction
Accurate identification and operation in the correct dissection plane are key factors for the successful preservation of critical structures during endocrine surgery. This preservation correlates with clinical outcomes and quality of life in these patients. In the past, inspection and palpation have been the main tools of a surgeon during surgery. Although all surgeons operate with careful dissection, there are still some limitations regarding organs or structural preservation in endocrine surgical procedures, especially parathyroid gland (PTG) preservation which we can see from the high incidence of postoperative parathyroid insufficiency in thyroid surgery (1) and uncertainty of parathyroid function after subtotal parathyroidectomy (2).
Indocyanine green (ICG) enhanced fluorescence imaging is one of the latest medical tools that uses intraoperative angiography with a fluorescent dye and is being applied by surgeons in order to overcome the above-mentioned limitations. The tool, thus, aims to verify the perfusion rates of PTGs. Previous research studies have shown the use of a particular technique or combination of techniques that have enabled surgeons to ensure the parathyroid remnant is adequately perfused, allowing for the total resection of the other PTGs to be carried out or to ensure that the PTGs are adequately vascularized after thyroid resection. This technique is used to reduce the chances of hypoparathyroidism after a patient undergoes thyroid surgery and can help to preserve postoperative parathyroid remnant function (2,3). We reviewed the current studies of ICG in endocrine surgery focusing on parathyroid preservation within this report.
The use of ICG enhanced fluorescence imaging
ICG can be defined as an amphiphilic tricarbocyanine dye with its molecular weight measured as 751.4 Dalton, a maximum absorption spectrum of 805 nm and with re-emission at 835 nm (4). Once introduced into the bloodstream, it becomes fully attached to plasmatic proteins and only circulates within the intravascular fluid compartment of the blood. This process enables it to act as a real-time intravascular contrast with a half-life of between 3–5 min and elimination happening after 15–20 min from the time of absorption. This is ultimately taken up by the hepatocytes and excreted by the biliary system. Generally, ICG is injected intravenously and further injections can be would reach a maximum toxic dose at a level of 5 mg/kg per day (5). Adverse reactions in patients who are treated are rare, with fatal allergic reactions occurring in 1/333,000 of cases (5).
ICG was originally developed for use in photography and later approved by FDA for clinical use in 1959, which led to its angiographic use as a technique to detect macular degeneration in patients (6). ICG has various advantages such as its excellent safety rating and its short life-time in terms of its presence within a patient’s bloodstream. With accessible imaging devices, it has been established for many clinical applications including assessing vessel patency during surgery (7,8), the delineation of tumor/metastasis after ICG is administered intravenously (9-11), and lymphatic architecture assessment after subcutaneous and intradermal ICG administration (12-14). It has been introduced into many fields and procedures of surgery, for example, in treating wounds and traumas, reconstructive operations, various neurosurgical procedures, bypass coronary surgery, and laparoscopic surgery (4,5,15).
Many devices have been employed in the published studies (4,15,16). There are several variables in terms of how each of the systems differ from one another, including such factors as the wavelength and bandwidth of excitation sources, required power input, and techniques employed for the filtration for the rejection of backscattered excitation and ambient light that influence the sensitivity levels of such device, as outlined in Table 1 (15).

Full table
Recently, ICG fluorescence imaging was used with endocrine surgery, including thyroid and parathyroid surgery. Its use has been studied by several groups (2,17-22). However, an overall consensus on the optimal dosage and timing of ICG administration is still lacking. Current applications of ICG enhanced fluorescence in thyroid and parathyroid surgery have been reviewed in this study.
ICG enhanced fluorescence in thyroidectomy
Syndromes of hypoparathyroidism after total thyroidectomy
One of the most common complications associated with total thyroidectomy is postoperative hypocalcemia (23). The estimated prevalence of transient hypoparathyroidism is from 19% to 38% and permanent hypoparathyroidism varies from 0% and 3%, respectively (23). The reason as to why the incidence of postoperative hypocalcemia reported a wide range of incidence is because of lack of any clear definition. The definition of hypoparathyroidism and hypocalcemia were varied across several studies that applied different timing of measurements, cut-off levels, and the addition of calcium and vitamin D supplements (24,25). Hypocalcemia has been defined with the range of the cut-off point between 1.8 to 2.12 mmol/L (23) and hypoparathyroidism reported when parathyroid hormone (PTH) less than 1.1 pmol/L. There was also variation in the cut-off period of transient and permanent hypoparathyroidism and temporary hypocalcemia which is less than 6 months or 1 year after total thyroidectomy.
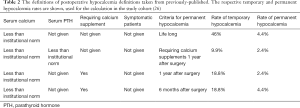
To date, one previous study focusing on the cohort series of 202 post-thyroidectomy patients in which ten alternative definitions were applied (with some examples and results as shown in Table 2), the results of post-thyroidectomy hypocalcemia could be 0–46% from this same group of patients (26).
Full table
Without the consensus of the definition, this could be the reason that explains the underestimated prevalence of postoperative parathyroid insufficiency and failure in general awareness, treatment, and prevention of post-thyroidectomy hypocalcemia.
A major cause of postoperative hypoparathyroidism is inadvertent injury during surgery, such as mechanical or thermal trauma, gland devascularization or incidental parathyroidectomy (23-25). There have been many studies that have reported the surgical risk factors of post-thyroidectomy hypocalcemia, such as re-operation, large goiter (25), Graves’ disease (27), and thyroid cancer (28). There have also been reports of clinical predictors of postoperative hypocalcemia, such as calcium levels being less than 1.88 mmol/L (reading taken 24 h after surgery), and the inability to identify more than one PTG during surgery (25).
Hypoparathyroidism has a wide range of impacts on patient’s quality of life, ranging from unexpected inability to control their hypoparathyroidism, other breakthrough symptoms despite current management regimens, and feelings of significant interference with their lives (29). Furthermore, there could be the potential for long-term complications in patients with permanent hypoparathyroidism, with risks including the development of renal failure, basal ganglia calcifications, neuropsychiatric derangements and infections (30,31).
Reducing and ultimately eliminating such complications after surgery can greatly improve a patient’s overall quality of life (23-25,29-31). Preservation of parathyroid function is the key factor to ensure better outcomes but, at the same time, also presents a challenge even for experienced surgeons. According to several studies, it has been concluded that despite the fact that PTGs are often considered to be well preserved at the time of surgery, postoperative parathyroid function cannot be guaranteed afterwards (32-34).
Many techniques and approaches have been proposed to improve the outcomes and prevent post-thyroidectomy hypocalcemia (18,20,22,35-40). Among these techniques, intraoperative ICG with fluorescence imaging has been one of the most promising methods for identifying and preserving parathyroid function (18,20,22).
Identification and perfusion assessment of PTGs with ICG enhanced fluorescence during thyroidectomy
Several groups have studied the role that ICG plays in helping surgeons to preserve PTGs from inadvertent injury and evaluate vasculature after thyroidectomy as shown in Figure 1.
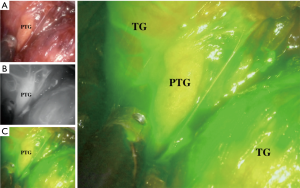
In 2016, Vidal Fortuny et al. reported a pilot study that evaluated the application of ICG angiography in an attempt to predict function levels of the PTG following thyroidectomy (18). Thirty-six patients undergoing total thyroidectomy were the subjects of this study. According to the protocols of the 25-mg dye, ICG was prepared with a mixture of 10 mL sterile water, while 3.5 mL of such mixture was intravenously injected following the completion of the thyroidectomy procedure. Images were taken with a laparoscopic PINPOINT® camera (Novadaq, Ontario, Canada) after 1 or 2 min. All identified PTGs with ICG enhanced fluorescence were graded on a visual basis (i.e., their subjective appearance to a surgeon’s naked eye) in terms of their viability. The grading system was from 0 (reported no or poor vascularity) to 2 (reported excellent vascularity). The mean duration of the ICG angiography procedure was 6±2 min, based on a mean average time reading.
Ninety-nine PTGs could be identified from these patients. One of the 30 (3.33%) patients with a minimum of one well vascularized gland was identified to have asymptomatic hypocalcemia and levels eventually was normalized at postoperative day (POD) 10. On the contrary, ICG angiography was not found to demonstrate good vascularization of the PTGs of six patients included in the study and a further two of the patients (one-third in total) developed transient hypoparathyroidism. It was found that no patients developed symptomatic hypocalcemia or required additional treatment using active vitamin D analogue.
In the same year, Zaidi et al. reported a different technique of ICG injection and grading system (20). This involved a total of 27 patients who were undergoing total thyroidectomy including for both benign and malignant conditions, with a Pinpoint video-assisted NIR system (Novadaq) used during the analyzed procedure. ICG was injected before and after the dissection of the thyroid gland, with the design of the study containing two different implications, in that:
- To identify the PTGs before the dissection of the thyroid gland through the use of this intra-operative imaging. This, in turn, could possibly reduce potential injury or contamination in the bloodstream as the area of dissection in relation to the thyroid gland could be pinpointed more accurately;
- To evaluate the viability of PTGs following total thyroidectomy through the use application of ICG fluorescence as an objective parameter with four varying degrees of ICG fluorescence present in PTGs following a thyroidectomy procedure; grade 0 (shows no ICG uptake), grade 1 (shows <30% uptake), grade 2 (shows 30–70% uptake), and grade 3 (shows >70% uptake) (20).
Through visual identification, a total of 85 glands could be identified, with a total of 71 (84%) showing the presence of ICG fluorescence. The degree of ICG fluorescence following the completion of the procedure was 3 (>70% of the volume of the gland) in 38/85 glands (44.7%); 2 (30–70%) in 26/85 (30.6%) glands; 1 (<30%) in 7/85 (8.2%) glands; and 0 (no uptake) in 14/85 (16.4%) glands.
Following the completion of each thyroidectomy, ICG uptake correlated with the levels of postoperative serum PTH. The mean average level (reported on the POD1 PTH of each patient with a minimum of two glands exhibiting <30% fluorescence at the time of completion of thyroidectomy surgery was 9 pg/dL; patients with one or no glands demonstrating <30% fluorescence were reported to have POD1 PTH of 19.5 pg/dL (P=0.05). POD1 serum calcium levels reported in such patients with two or more under-perfused glands did not significantly differ between patients with fewer than two under-perfused glands. A total of three patients (11% of all subjects) were reported to have a serum calcium value of <8 mg/dL and one was exhibiting symptoms associated with such treatment.
In a recent study, Yu et al. combined ICG enhanced fluorescence imaging with a bilateral axillo-breast approach (BABA) robotic thyroidectomy (RoT) (22) in which a total of 11 patients were undergoing total thyroidectomy and the same number (11) undergoing thyroid lobectomy with the application of ICG. The Firefly system (NIR illuminator: 805 nm; filter: 825 nm), used in conjunction with ICG fluorescence, was integrated into the da Vinci Si robot system with the aim of identifying the patients’ lower PTGs. In order to accurately know that the ICG injection was indeed illuminating PTGs rather than any other internal structure, 9 of the 11 patients were subjected to an intraoperative tissue PTH assay. The average range of time from the time of injection to the visualization of the inferior parathyroid and thyroid gland by using ICG fluorescence were between 203±89 [125–331]. The fluorescence appeared (illumination) in the glands for an average time range of 20.8±6.0 (16.6–35.8) and 20.1±7.3 (15.5–33.8) min. It was found that the control group (0% vs. 15.9%, P=0.048) demonstrated a significantly higher rate of incidental parathyroidectomy than that of the ICG group, which had comparatively lower rates. However, result shows the lack of statistical significance regarding the rates of transient and permanent hypoparathyroidism (22).
Later, Lang et al. conducted a study of ICG fluorescence imaging to help to predict postoperative parathyroid function in 94 patients after open total thyroidectomy (21). The SPY Fluorescent Imaging system (Novadaq) was used in the study to perform Intraoperative ICG angiography. The emitted fluorescence from the SPY Fluorescent Imaging system was recorded in terms of its intensity (defined as the “FI” range). The measurement technique used a charge-coupled camera setup to directly reflect perfusion levels within that highlighted region in which PTGs and anterior trachea were compared.
In this study, they were able to find 4 PTGs in 70 patients. Out of these, a total of nine (equivalent to 12.9%) of the total number of patients developed post-thyroidectomy hypocalcemia. All (100%) of the patients had no problems in discontinuing the use of calcium and vitamin D supplements within a time period of 3 months following their operation, nor did any exhibit symptoms of hypocalcemia or develop permanent hypocalcemia. In the same study, no occurrences of any parathyroid tissue or gland being inadvertently discovered on the excised thyroid specimen were reported.
The median (range) of the highest FI level of each patient was 208.50% (105.00–419.00%) and the median average range of the FI levels of each patient was recorded at 155.80% (55.50–320.00%). It was found that significant correlations existed between the highest FI level and PTH-D0 (r=0.081, P<0.001), the highest FI level and PTH-D1 (r=0.598, P<0.001) and the highest FI level and percentage of reduction in PTH from the preoperative to day-0 (r=0.239, P<0.001). However, there was not a significant correlation between the average FI level and PTH-D0 (r=0.109, P=0.371), the average PTH-D1 (r=0.106, P=0.384) and the average FI level and the percentage of reduction in PTH recorded from the preoperative to day-0 (r=0.115, P=0.345).
In the case where patients had the highest FI value >150%, the statistical probability of developing postoperative hypocalcemia was 0 (0/59), and also in the case where the highest FI value was <150%, 9 (81.8%) developed PH. In the case of the two patients with the highest FI value <150% and who did not develop postoperative hypocalcemia, the highest FI values were recorded at 116% and 119%, respectively. These patients’ average FI values were recorded at 56.25% and 112.0%, respectively. In this same light, in cases where a patient had an average FI recorded at >109%, the statistical probability of such patient developing postoperative hypocalcemia was 0 (0/40); however, in cases in which the overall average FI was recorded at <109%, 9/30 (30.0%), patients did develop postoperative hypocalcemia.
Based on this data, the study group has proposed the highest FI values of (<150% and >150%) to be used for predictive tests of postoperative hypocalcemia. This is because these values having the highest levels of sensitivity and specificity, while they are also predictability relative to the other tests. In addition, the highest FI level recorded was superior to PTH-D0 (P=0.027) and the percentage of PTH drop from day 0 to day 1 (P<0.001). In addition, while perhaps not as important, the highest FI level recorded was usually superior to the average FI level (P=0.073).
From many published reports, we believe that the utility of this ICG enhanced fluorescence imaging has promising benefits but there was variation across studies as shown in Table S1. One of the drawbacks of the current studies are the sample sizes, which are too small to clearly identify the correlation of well-vascularized PTGs and post-thyroidectomy parathyroid function. The lack of quantitative measurements of PTG vascularization (but rather a basis which relies on surgeons’ subjective decisions) is an important limitation to compare the results between studies and impacts on the reliability of methodology. We believe that a randomized controlled trial comparing the use of ICG enhanced fluorescence imaging and naked eye with intraoperative confirmation of PTGs and quantitative measurement vascularization should be conducted to validate the follow-up studies.

Full table
ICG enhanced fluorescence in parathyroidectomy
Although ultrasound and sestamibi scan can be used to guide parathyroidectomy, their sensitivities are around 69–75% and 49–70%, respectively (41). There are still limitations of applying these localizations into the real-time procedures especially for identification of the PTG. Many techniques have been reported to be able to overcome this limitation (42-44).
Among these techniques, ICG enhanced fluorescence imaging is one of the most feasible and promising methods to improve the localization of PTGs and help surgeons to evaluate perfusion and predict its preserve function after parathyroidectomy intraoperatively. From the published studies, ICG was used with the indications as follows:
- Identification and localization of pathologic PTGs in primary hyperparathyroidism, especially in redo-operation (17,19,45);
- Evaluation perfusion of preserved PTGs and prediction of its function postoperatively with the aim of avoiding long-term hypoparathyroidism with low levels of PTH immediately after parathyroidectomy after subtotal parathyroidectomy in renal hyperparathyroidism or other indications (2).
In 2016, Vidal Fortuny et al. (2) described their use of ICG angiography used to evaluate how parathyroid remnant in subtotal parathyroidectomy functions, with this particular study comprised of 13 patients undergoing treatment. With a dose of 5 mg/kg, each patient was intravenously injected with ICG once per day. However, the study did not specifically record the exact dosage amount for each patient (but, rather, was based on the above-mentioned ratio of 5 mg/kg). A Pinpoint Endoscopic Fluorescence Imaging System (Novadaq) was enabled for the visualization of gland perfusion of the course of the study period. A visual color scale that used grey or green tones (depending on the mode of viewing) was used to define the perfusion of the glands and the remnant. The mean duration of performing the ICG angiography was 3.0±2.3 min. According to the results obtained by the ICG angiography technique, the parathyroid remnant that was most effectively perfused could be successfully identified. Ten patients in the study underwent the removal of 31⁄2 glands while three patients had three glands removed. Metallic clips were used for half-gland resection during the procedure. In the short-term follow ups, it was found that the previously corrected calcium and PTH levels could be defined as being in a ‘normal’ range for 100% (13 in total) of the patients. This assumption was based on the fact that their pathology and PTH levels had dropped from 70.7%±22.5% in comparison to the level taken before the operation.
Conclusions
This study reviews the current status of ICG enhanced fluorescence imaging and parathyroid preservation in both thyroid and parathyroid surgery. Although there still are questions regarding its utility, current data suggests that a correlation does exist with regards to the relationship between parathyroid perfusion and postoperative parathyroid function. In light of this, additional studies are necessary for the further validation of ICG angiography as an intraoperative tool in assessing real-time parathyroid preservation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thomusch O, Machens A, Sekulla C, et al. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003;133:180-5. [Crossref] [PubMed]
- Vidal Fortuny J, Sadowski SM, Belfontali V, et al. Indocyanine Green Angiography in Subtotal Parathyroidectomy: Technique for the Function of the Parathyroid Remnant. J Am Coll Surg 2016;223:e43-9. [Crossref] [PubMed]
- Kahramangil B, Berber E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol 2017;115:848-55. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [PubMed]
- Vidal Fortuny J, Karenovics W, Triponez F, et al. Intra-Operative Indocyanine Green Angiography of the Parathyroid Gland. World J Surg 2016;40:2378-81. [Crossref] [PubMed]
- de Boer E, Harlaar NJ, Taruttis A, et al. Optical innovations in surgery. Br J Surg 2015;102:e56-72. [Crossref] [PubMed]
- Taggart DP, Choudhary B, Anastasiadis K, et al. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg 2003;75:870-3. [Crossref] [PubMed]
- Sekijima M, Tojimbara T, Sato S, et al. An intraoperative fluorescent imaging system in organ transplantation. Transplant Proc 2004;36:2188-90. [Crossref] [PubMed]
- Miyashiro I, Miyoshi N, Hiratsuka M, et al. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol 2008;15:1640-3. [Crossref] [PubMed]
- Tagaya N, Yamazaki R, Nakagawa A, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg 2008;195:850-3. [Crossref] [PubMed]
- Noura S, Ohue M, Seki Y, et al. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a nearinfrared camera system. Ann Surg Oncol 2010;17:144-51. [Crossref] [PubMed]
- Rasmussen JC, Tan IC, Marshall MV, et al. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol 2009;20:74-82. [Crossref] [PubMed]
- Zhang J, Zhou SK, Xiang X, et al. Automated analysis of investigational near-infrared fluorescence lymphatic imaging in humans. Biomed Opt Express 2012;3:1713-23. [Crossref] [PubMed]
- Groenlund JH, Telinius N, Skov SN, et al. A Validation Study of Near-Infrared Fluorescence Imaging of Lymphatic Vessels in Humans. Lymphat Res Biol 2017;15:227-34. [Crossref] [PubMed]
- Marshall MV, Rasmussen JC, Tan IC, et al. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg Oncol J 2010;2:12-25. [Crossref] [PubMed]
- Lavazza M, Liu X, Wu C, et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg 2016;5:512-21. [Crossref] [PubMed]
- Chakedis JM, Maser C, Brumund KT, et al. Indocyanine green fluorescence-guided redo parathyroidectomy. BMJ Case Rep 2015;2015:bcr2015211778. [Crossref] [PubMed]
- Vidal Fortuny J, Belfontali V, Sadowski SM, et al. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016;103:537-43. [Crossref] [PubMed]
- Zaidi N, Bucak E, Okoh A, et al. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol 2016;113:771-4. [Crossref] [PubMed]
- Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775-8. [Crossref] [PubMed]
- Lang BH, Wong CK, Hung HT, et al. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017;161:87-95. [Crossref] [PubMed]
- Yu HW, Chung JW, Yi JW, et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg Endosc 2017;31:3020-7. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Munoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015;4:82-90. [PubMed]
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and metaanalysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2010;32:279-83. [PubMed]
- Pesce CE, Shiue Z, Tsai HL, et al. Postoperative hypocalcemia after thyroidectomy for Graves' disease. Thyroid 2010;20:1279-83. [Crossref] [PubMed]
- Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg 2007;245:604-10. [Crossref] [PubMed]
- Hadker N, Egan J, Sanders J, et al. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the PARADOX study. Endocr Pract 2014;20:671-9. [Crossref] [PubMed]
- Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507-14. [Crossref] [PubMed]
- Leidig-Bruckner G, Bruckner T, Raue F, et al. Long-Term Follow-Up and Treatment of Postoperative Permanent Hypoparathyroidism in Patients with Medullary Thyroid Carcinoma: Differences in Complete and Partial Disease. Horm Metab Res 2016;48:806-13. [Crossref] [PubMed]
- Song CM, Jung JH, Ji YB, et al. Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol 2014;12:200. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359-67. [Crossref] [PubMed]
- Moley JF, Skinner M, Gillanders WE, et al. Management of the Parathyroid Glands During Preventive Thyroidectomy in Patients With Multiple Endocrine Neoplasia Type 2. Ann Surg 2015;262:641-6. [Crossref] [PubMed]
- Bian XH, Li SJ, Zhou L, et al. Applicability of rapid intraoperative parathyroid hormone assay through fine needle aspiration to identify parathyroid tissue in thyroid surgery. Exp Ther Med 2016;12:4072-6. [Crossref] [PubMed]
- Cui Q, Li Z, Kong D, et al. A prospective cohort study of novel functional types of parathyroid glands in thyroidectomy: In situ preservation or auto-transplantation? Medicine (Baltimore) 2016;95:e5810. [Crossref] [PubMed]
- Falco J, Dip F, Quadri P, et al. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J Am Coll Surg 2016;223:374-80. [Crossref] [PubMed]
- Kim SW, Song SH, Lee HS, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. J Clin Endocrinol Metab 2016;101:4646-52. [Crossref] [PubMed]
- Park I, Rhu J, Woo JW, et al. Preserving Parathyroid Gland Vasculature to Reduce Post-thyroidectomy Hypocalcemia. World J Surg 2016;40:1382-9. [Crossref] [PubMed]
- Hou F, Yu Y, Liang Y. Automatic identification of parathyroid in optical coherence tomography images. Lasers Surg Med 2017;49:305-11. [Crossref] [PubMed]
- Siperstein A, Berber E, Barbosa GF, et al. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg 2008;248:420-8. [PubMed]
- Prosst RL, Gahlen J, Schnuelle P, et al. Fluorescence-guided minimally invasive parathyroidectomy: a novel surgical therapy for secondary hyperparathyroidism. Am J Kidney Dis 2006;48:327-31. [Crossref] [PubMed]
- Grubbs EG, Mittendorf EA, Perrier ND, et al. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am J Surg 2008;196:931-5. [Crossref] [PubMed]
- Tummers QR, Schepers A, Hamming JF, et al. Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and lowdose Methylene Blue. Surgery 2015;158:1323-30; discussion 935-6. [Crossref] [PubMed]
- Sound S, Okoh A, Yigitbas H, et al. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg Innov 2015. [Epub ahead of print].


