Oncoplastic breast conserving surgery with tailored needle-guided excision
Introduction
Oncoplastic breast conserving surgery (OBCS) is the best option for early breast cancer aiming to achieve complete tumor excision with no involved surgical margins, which currently means “no ink on tumor” (1), and good objective cosmetic outcome. Many techniques are used to help surgeons to carry out a complete tumor excision, but needle-wired localization (WL), which is named “bracketing” when multiple wires are inserted, is one of the most commonly employed (2). We described our WL technique, which helps surgeons by marking the limits of the resection, inserting some wires 1 cm distance to radiological lesion limits, and by warning them of conflictive points which could compromise surgical technique (3).
There is a lack of publications about “bracketing” in the context of oncoplastic approach and some authors (4,5) have reported that the use of multiple needles (bracketing) to localize neoplasms was associated with higher positive margins than when a single needle was required.
We report our experience in the surgical treatment of early breast cancer combining OCBS and tailored WL and the analysis of factors which are related to positive margins.
Methods
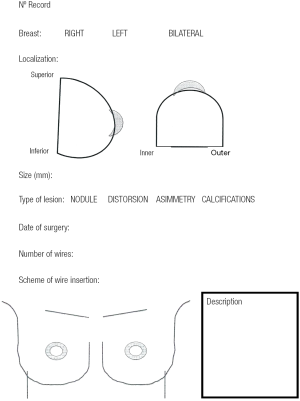
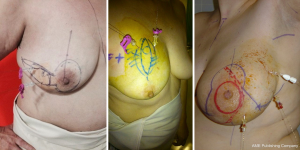
We reviewed the records of 148 patients with breast cancer who were treated with OBCS and WL in the Breast Unit of Hospital Valdecilla (Santander, Spain) from March 2013 to December 2015. At the Radiological Department, where all image data are available, the surgeon and radiologist decide and fill out the diagram (Figures 1,2) showing how many wires and where should be inserted. The diagram shows data concerning the affected breast, in which quadrant the lesion is located, the type of radiological lesion (nodule, distortion, microcalcifications and asymmetry), the maximum diameter of the tumor or the distance comprising the entire lesion to be removed, the day of the surgery, the number of wires and two drawings of lateral and craniocaudal mammograms where the location of the lesion and the situation of the wires can be drawn. The day of the surgery the diagram is used by the radiologist to insert the wires in the place and in the way agreed on. Wires can be inserted into the breast laterally or perpendicularly to the chest wall. This choice is mainly determined by the oncoplastic incision pattern to be used and the location of the lesion. For example, if we are going to perform a “diamond”, “round block” or “batwing mammaplasty” incisions we prefer perpendicular insertions, whereas if we are going to carry out a therapeutic mammaplasty with an inverted T-incision pattern or “tennis racket” incision, a lateral insertion is preferred (Figure 3). However, the localization and the way in which the wires should be inserted are decided depending on the particularities of each case. In the diagram each path is drawn in a different way, lateral or perpendicular (←(x) respectively.
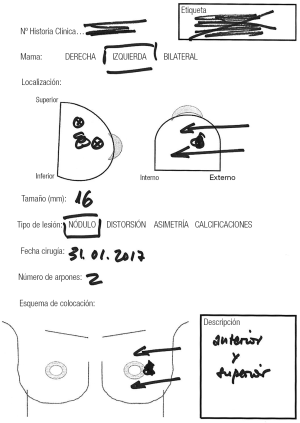
The number of wires used for localization was: 1 in 52 patients, 2 in 88, 3 in 7 and 4 in a patient with a bilateral cancer.
All patients were operated on by two surgeons (Fernando Hernanz and Mónica González-Noriega) who planned and performed surgical procedures working together on the same patient most of the time. The resection was performed outside the wires and sticking to them, often two, which were located at two different points crossing orthogonally at the center of the lesion, so the surgeon had to calculate the limits of the resection by comparing the location of the two wires and thinking that they were 1 cm away from the radiological limits of the lesion (Figure 4). In patients with macromastia treated with therapeutic mammoplasty, as a large amount of breast tissue was removed, a wire was used to locate the lesion to avoid its being left in the breast.
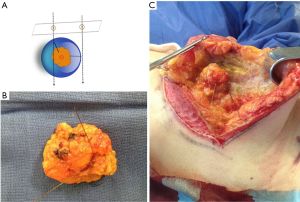
After the resection was carried out, the surgical specimen was marked at superior and medial sides with some stitches (2 long and 1 short, respectively) in order to guide the pathologist, and then sent to the Radiological Department where two orthogonal digital mammograms were taken. The radiologist informed the surgeon whether the entire radiological lesion was included in the surgical specimen and if it was closed to any side. In this case, the surgeon shaved the close margin with a scalpel. Digital images of the surgical specimen could be visualized by the surgeon in the operating theatre to check the radiological margins status and to decide, with the radiologist’s report, how to do margin extension. Before remodeling the breast, all four sides of the breast cavity were marked with titanium clips to facilitate radiation therapy.
Pathologic slides of the patients whose reports were informed as not having free margins in the breast tissue removed, were revised by a pathologist (MH) applying the “no ink on tumor” consensus guideline on margins for breast-conserving surgery published on March 2014 (1) because some cases had been evaluated before this criteria was released.
Statistical analysis
Firstly, we described the distribution of clinical and pathological variables in the series of patients. Secondly, we compared the distribution of categorical variables between two groups, with or without affected margins, with chi-square or Fisher tests and numerical variables with Mann-Whitney test. Lastly, Univariate and multivariate logistic regression analyses with the method enter were performed to test for association between clinic-pathologic variables and positive resection margins in 133 patients with invasive non bilateral breast cancer. Four variables were transformed: age (<50 or ≥50 years), radiologic tumor size (<20 or ≥20 mm) histologic subtype (lobular and no-lobular), and intrinsic subtype luminal B_Her2 and Her2 became one. In categorical variables the category with lower risk was considered to be the variable of reference. Analysis was performed with MedCalc Statistical Software version 17.4 (MedCalc Software BVBA, Ostend, Belgium; http://www.medcalc.org; 2017). A P value of >0.05 was considered statistically significant.
Results
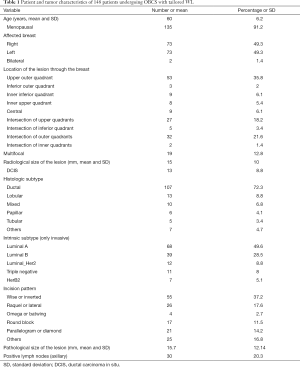
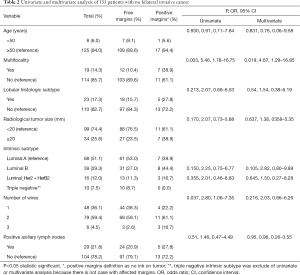
Demographic characteristics of the patients are described in Table 1. Twenty patients had involved surgical margins (13.5%), 7 with invasive cancer, 11 with ductal carcinoma in situ (DCIS) component, and 2 both. Seventeen patients were re-operated on, 16 for oncologic reason (10.8%), 12 with affected margins having also 2 of them positive sentinel lymph node, and another 4 for positive sentinel lymph node. We performed 3 re-excisions, 9 mastectomies and 6 lymphadenectomies. The final rate of BCS was 94%. Five (41.6%) patients had residual cancer in the breast tissue and only two had more positive lymph nodes. A woman was operated on due to a surgical complication (hemorrhage). Table 2 shows results of logistic regression analysis.
Full table
Full table
Discussion
The majority of our patients were referred from a breast cancer screening program so their ages were over 50 (average of 60); they were menopausal (91%) having non-palpable cancer with 8.8% of DCIS. Invasive cancer was most frequently luminal A and B (78%). Despite early diagnose, the radiological size average was 15 mm (9.9 SD), which had a very high correspondence with the pathological one, 15.8 mm (12.1 SD), and 20.3% had positive axillary lymph nodes. Therapeutic mammoplasty was the oncoplastic technique most frequently used with 37% of patients; in our opinion, this technique is very versatile and can be used in all quadrants on condition that the breast is medium or large-sized, or has enough degree of ptosis (6).
Bracketing comprises using two or more needles for localization of boundaries of an impalpable breast lesion. The tissue limited by them is excised and sent for histopathology. We introduce a slight modification which consists of using needles not only to localize the lesion, but also to mark the limits of the resection by inserting them at 1 cm distance to the radiological limits with the purpose of performing an accurate resection with free radiological margins. Like Tardioli et al. (7), who coined the term “optimized wire-guided localization” and also treated their patients with oncoplastic techniques, we consider essential discussing with the radiologist the number of wires, the point and the way they should be inserted, taking into account the particularities of each case (type of radiological lesion, localization through the breast, incision patterns and OCBS technique, histological type of the tumor) tailoring the surgical approach for each patient.
Another modification is used when the oncoplastic technique requires NAP mobilization by a flap and the tumor is localized in retroareolar region. In this case, we use wires to warn the surgeon of conflictive points, such as retroareolar space, with the aim to preserve an appropriate width of the flap and get a complete tumor excision. WL is very common and it is available in almost every center. However, it has some disadvantages. It is time-consuming and disturbs and hurts patients.
We obtained a 13.5% rate of involved surgical margins by combining our tailored WL and an oncoplastic approach. This approach allows the resection of a large amount of breast tissue without compromising cosmetic outcome and which avoids dislocation of wires or their accidental section because tunneling is easier than conventional techniques. This rate is lower than others recently published, which analyzed large series of patients from population registers and similar to others OBCS series.
van Deurzen CHM (8) reported a 16.8% rate of affected margins in a population-based cohort study with data from The Dutch Pathology Register between 2009 and 2015, which consisted of a huge number of patients suffering from an invasive breast cancer [25, 315] who were treated by BCS. The multivariate logistic regression analysis found that multifocal location, lobular subtype, large tumor size and the presence of DCIS were strongly associated with involved margins [odds ratio (OR) >2].
Langhans (9) reported a positive margins rate and reoperation (17.6%) using wire-guided BCS in invasive and in situ ductal carcinomas in a large series of patients (4,118 women) analyzing data from Danish National Patient Registry during a period of 4 years (2010 to 2013); they found that DCIS increases the risk of affected margins 3 times over invasive cancer.
Haloua et al. (10) reported a 16.4% rate of involved surgical margins after BCS in a study which collected data from a Netherland network from 2012 (9,276 pathology excerpts). Laws et al. (11) communicated an overall positive margin rate of 20.8% in 1,165 patients from a database which captures 95% breast surgeries in Alberta (Canada).
Since the end of 1900s, when oncoplastic approach began, it has been spreading over breast units, and it has increased notably in the last decade; as an example of this, Carter et al. (12) state that the use of oncoplastic breast surgery experimented a nearly fourfold increase in the percentage of all breast cancer surgeries during the study period (2007 to 2014) in a single center study comprising 10,607 operations; 75% of the patients had an early cancer (T1 or T2 tumor) and the rate of positive or close margins was lower for oncoplastic techniques than conventional ones (5.8% vs. 8.3%).
Although it is really certain that OBCS allows carrying out a wide resection with a small alteration of breast cosmetic outcome, and consequently the rate of affected margins is lower than conventional BCS (13-15), there are many different techniques and their application is not uniform with a heterogeneous patient selection. Therefore, articles about OBCS are assorted and show a great variation of involved surgical margins rate (0 to 36%) (16,17).
Fitoussi et al. (18) in a large series of 540 patients who were treated with oncoplastic techniques using both volume replacement and displacement ones, obtained 18.9% of close or affected surgical margins with a 9.4% mastectomies. Clough (19), one of the pioneers of oncoplastic approach, in a total of 277 level II oncoplastic techniques performed on 272 patients, reported a rate of 11.9% positive margins with invasive lobular carcinoma as a variable with higher risk of positive margins.
De la Cruz et al. (20) reviewed eleven articles on OBCS comprising 1,455 patients and found a very low rate of 7.8% with “no ink on tumor criteria”, thus confirming the oncological safety of these procedures in patients with early invasive breast cancer.
Some clinical-pathological variables which can be assessed before surgery by imaging and needle biopsy, such as invasive lobular histologic subtype, large tumor size, presence of DCIS or microcalcifications on mammography, number of wires, etc. have been related to the increase of involved margins in many different studies. In our work, only multifocality, which may be the most common, increased heavily the risk of positive surgical margins. However, conservative surgery was possible in 63% of the cases with multifocal tumors.
Conclusions
In our experience, tailored WL, which requires collaborative working with the radiologist, helps the surgeon to carry out a theoretic breast tissue resection at 1 cm distance to the radiological limits of the lesion increasing the chance of obtaining pathologic free margins. Combining both approaches we obtained an acceptable rate of involved surgical margins, which is in the lower band of the range of data published, and high final rate of BCS. According to our finding, surgeons should be aware of the great risk of affected surgical margins in multifocal breast cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Access to patient data was according to permission rules from the Admission Department and Archive of our hospital (Hospital “Marqués de Valdecilla”, Santander) and patient confidentiality was rigorously preserved. Written informed consent was obtained from patients for publication accompanying images.
References
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 2014;21:704-16. [Crossref] [PubMed]
- Chan BK, Wiseberg-Firtell JA, Jois RH, et al. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev 2015.CD009206. [PubMed]
- Hernanz F, Regaño S, Vega A, et al. Needle-wire-guided breast tumor excision. J Surg Oncol 2006;94:165-6. [Crossref] [PubMed]
- Edwards SB, Leitman IM, Wengrofsky AJ, et al. Identifying Factors and Techniques to Decrease the Positive Margin Rate in Partial Mastectomies: Have We Missed the Mark? Breast J 2016;22:303-9. [Crossref] [PubMed]
- Kim SH, Cornacchi SD, Heller B, et al. An evaluation of intraoperative digital specimen mammography versus conventional specimen radiography for the excision of nonpalpable breast lesions. Am J Surg 2013;205:703-10. [Crossref] [PubMed]
- de la Fuente H, Luis F, Noriega G, et al. Versatility of therapeutic reduction mammoplasty in oncoplastic breast conserving surgery. World J Surg Proced 2015;5:217-22. [Crossref]
- Tardioli S, Ballesio L, Gigli S, et al. Wire-guided Localization in Non-palpable Breast Cancer: Results from Monocentric Experience. Anticancer Res 2016;36:2423-7. [PubMed]
- van Deurzen CH. Predictors of Surgical Margin Following Breast-Conserving Surgery: A Large Population-Based Cohort Study. Ann Surg Oncol 2016;23:627-33. [Crossref] [PubMed]
- Langhans L, Jensen MB, Talman MM, et al. Reoperation Rates in Ductal Carcinoma In Situ vs Invasive Breast Cancer After Wire-Guided Breast-Conserving Surgery. JAMA Surg 2017;152:378-84. [Crossref] [PubMed]
- Haloua MH, Volders JH, Krekel NM, et al. A nationwide pathology study on surgical margins and excision volumes after breast-conserving surgery: There is still much to be gained. Breast 2016;25:14-21. [Crossref] [PubMed]
- Laws A, Brar MS, Bouchard-Fortier A, et al. Intraoperative Margin Assessment in Wire-Localized Breast-Conserving Surgery for Invasive Cancer: A Population-Level Comparison of Techniques. Ann Surg Oncol 2016;23:3290-6. [Crossref] [PubMed]
- Carter SA, Lyons GR, Kuerer HM, et al. Operative and Oncologic Outcomes in 9861 Patients with Operable Breast Cancer: Single-Institution Analysis of Breast Conservation with Oncoplastic Reconstruction. Ann Surg Oncol 2016;23:3190-8. [Crossref] [PubMed]
- Down SK, Jha PK, Burger A, et al. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast J 2013;19:56-63. [Crossref] [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- Crown A, Wechter DG, Grumley JW. Oncoplastic Breast-Conserving Surgery Reduces Mastectomy and Postoperative Re-excision Rates. Ann Surg Oncol 2015;22:3363-8. [Crossref] [PubMed]
- Yiannakopoulou EC, Mathelin C. Oncoplastic breast conserving surgery and oncological outcome: Systematic review. Eur J Surg Oncol 2016;42:625-30. [Crossref] [PubMed]
- Haloua MH, Krekel NM, Winters HA, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg 2013;257:609-20. [Crossref] [PubMed]
- Fitoussi AD, Berry MG, Famà F, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases Plast Reconstr Surg 2010;125:454-62. [outcomes article]. [Crossref] [PubMed]
- Clough KB, Gouveia PF, Benyahi D, et al. Positive Margins After Oncoplastic Surgery for Breast Cancer. Ann Surg Oncol 2015;22:4247-53. [Crossref] [PubMed]
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]