Parathyroid transplantation in thyroid surgery
Introduction
Hypoparathyroidism is a common complication following bilateral thyroid surgery. Temporary hypoparathyroidism occurs in approximately 10% to 30% of patients, whereas permanent hypoparathyroidism rates are much less common being reported in 1% to 3% of patients (1). The risk of hypoparathyroidism increases with the extent of thyroidectomy, completion thyroidectomy, reoperative procedures and central neck clearance (1,2). Parathyroid identification and preservation is the mainstay of safe thyroid surgery. Efforts should be made to identify intraoperatively all four parathyroid glands. Use of a meticulous surgical technique and dissection close to the thyroid gland aiming at preservation of the intact blood supply to the parathyroids, and keeping away from unnecessary manipulation of parathyroids in due course of the dissection are well established factors contributing to the reduced prevalence of temporary and permanent hypocalcemia (1). However, if the parathyroid glands are damaged, autotransplantation should be undertaken to preserve their function. Implanted glands have a more predictable survival compared to devitalized glands that are left in situ (3).
In general, parathyroid transplantation can be considered in three distinct modes of application:
- Fresh parathyroid tissue autotransplantation during thyroidectomy in order to reduce the risk of permanent hypoparathyroidism;
- Cryopreserved parathyroid tissue autotransplantation in patients with permanent hypocalcemia;
- Parathyroid allotransplantation in patients with permanent hypoparathyroidism when cryopreserved parathyroid tissue is not available for grafting.
This paper is aimed to provide a review of current status of various parathyroid transplantation techniques in thyroid surgery.
Fresh parathyroid tissue autotransplantation
In 1975, Wells et al. reported on a successful parathyroid autotransplantation in a large cohort of patients undergoing parathyroidectomy with a transplant success rate of 93% (4). This study opened the way for further interest in reimplantation as an effective method of preservation of parathyroid function not only in parathyroid surgery but also in thyroid surgery, and especially in patients undergoing total thyroidectomy. Parathyroid autotransplantation soon became a widely accepted technique in head and neck surgical oncology and endocrine surgery as an effective way to reduce the risk of permanent postoperative hypocalcemia (5).
Selective versus routine parathyroid autotransplantation
Indications for parathyroid autotransplantation during thyroid surgery are: an inadvertent removal of parathyroid gland, finding parathyroid in the surgical specimen soon after excision, no anatomical capacity for preservation of the parathyroid in situ, and devascularization of the parathyroid gland. In such cases parathyroid should be reimplanted into the sternocleidomastoid muscle (6). There are several techniques in the surgical armamentarium allowing for intraoperative determination of parathyroid viability. The most commonly adopted techniques are: observation of parathyroid color change, and an incisional test to verify vitality by inspection of capsular bleeding (7,8). It was also proposed to utilize the intraoperative parathyroid hormone assay (IOPTH) during thyroidectomy in monitoring of parathyroid function and this method turned out to be of added-value for the surgical decision making with respect to the selective parathyroid tissue reimplantation (9,10). Other concepts, such as routine reimplantation of at least one parathyroid gland, aiming at abolishing the prevalence of permanent hypoparathyroidism can be taken into account, but are associated with a markedly higher prevalence of transient hypoparathyroidism (9). Paloyan et al. reported on a series of 118 patients in whom parathyroid autotransplantation was increasingly utilized from one-fourth to the vast majority of patients undergoing thyroidectomy over consecutive years of the study period (11). In general prevalence of permanent hypocalcemia was 1.7%. However, the incidence of permanent hypoparathyroidism decreased from 3% in the beginning of the study to 0% by the end of the study when elective parathyroid autotransplantation was used in almost all patients. Moreover, Wilson suggested that routine reimplantation of at least one parathyroid gland is more reliable than leaving the parathyroid in situ even if the vascularization seemed to be unaltered because of a higher potential of the autograft to produce an expected functional outcome preventing the patient form permanent hypoparathyroid state (12).
Autotransplantation techniques
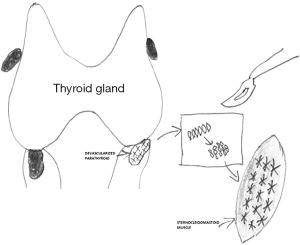
Parathyroid autografts can be placed heterotopically in a brachioradialis muscle or in the sternocleidomastoid muscle. However, the latter one is preferred during thyroid surgery, as additional incision can be avoided and because measurement of parathyroid hormone (PTH) gradient is not necessary afterwards (1). Before autotransplantation, a frozen section of a small fragment of the parathyroid gland should be made, particularly in patients operated for thyroid malignancy (13). Different methods of parathyroid autotransplantation have been described including the techniques of slicing, mincing, and injecting a solution of suspended parathyroid tissue in saline into the muscle (14). The widely accepted technique for hyperplastic parathyroid tissue reimplantation was described by Wells et al. (4). Parathyroid tissue should be stored for a while in a chilled saline soon after excision. After cooling for 30 min, the parathyroid glands become firm and can be easily sliced into 1-mm pieces or 1 mm × 1 mm × 1 mm cubes. In generally, 10–20 pieces should be inserted into separate muscle pockets of the brachioradialis or sternocleidomastoid muscle (Figure 1) (4). This technique allows to minimize the chances of graft malfunction. Any intramuscular hematoma formation may compromise the graft acceptance. Gauger et al. reported that an injection of a parathyroid tissue suspension in saline was as effective as sectioning or mincing of parathyroid gland for autotransplantation in patients undergoing thyroid surgery (15). Most patients after parathyroid autotransplantation require temporary oral calcium and vitamin D supplementation and close biochemical monitoring of serum calcium for 2–4 weeks following surgery. Utilizing such an approach markedly decreases the incidence of permanent postsurgical hypoparathyroidism (6,9). In the majority of published series, proof of successful parathyroid autotransplantation during thyroid surgery has been recognized based on the maintenance of normocalcaemia without need for life-long calcium supplementation after experiencing a short-term period of postoperative hypocalcaemia (16,17). However, in more recent papers it was also confirmed by PTH serum level measurements in peripheral blood (10,15).
IOPTH during thyroidectomy for selective parathyroid autotransplantation
An intraoperative assessment of parathyroid function was reported to be useful in identification of parathyroid glands with a degree of impaired vascularity, the method which has a potential to facilitate the decision to perform autotransplantation (9). In a randomized controlled trial of 340 patients undergoing total thyroidectomy Barczyński et al. found that IOPTH offered clinically important information during thyroid surgery, allowing for correct identification of patients with increased risk of postoperative hypocalcemia. Selective IOPTH-based parathyroid autotransplantation in patients with serum PTH levels below 10 ng/L at 10 to 20 minutes after total thyroidectomy abolished the risk of permanent hypoparathyroidism, and this concept turned out to be as effective as elective parathyroid autotransplantation of at least one parathyroid gland without IOPTH monitoring. In addition, selective IOPTH-based parathyroid reimplantation markedly reduced prevalence of transient postoperative hypocalcemia and the overall doses of calcium slats used for supplementation in this study arm were lower when compared to elective parathyroid autotransplantation without IOPTH monitoring (10).
Cryopreserved parathyroid tissue autotransplantation
Direct reimplantation of parathyroid glands that are damaged or inadvertently removed minimizes the risk of graft non viability and non-functionality. However, immediate autotransplantation may not be necessary in all patients undergoing thyroidectomy as in most subjects who experience hypocalcemia postoperatively this state is transient. Hence, cryopreservation of parathyroid tissue, the concept that was developed by Wells et al. to cope with a problem of permanent hypoparathyroidism, can be an alternative (4). This method allows for the storage of parathyroid tissue and subsequent autotransplantation in a delayed manner after the diagnosis of permanent hypothyroidism is confirmed (18). The physician is able to determine more accurately if there is any residual parathyroid tissue left behind with a potential of regaining functioning or whether delayed autotransplantation is needed (19). However, parathyroid tissue cryopreservation entails the potential risk of transplant failure or malfunction which is difficult to predict (3).
Indications for cryopreservation
The obvious indication for autotransplantation of cryopreserved parathyroid tissue is permanent iatrogenic postsurgical hypoparathyroidism. The prevalence of this adverse sequelae following thyroidectomy in a virgin neck is below 1–2% if only the operation is performed by an experienced thyroid surgeon (18). The risk of permanent hypoparathyroidism is considered to be minimal for a thyroidectomy alone but increases with more radical extent of surgery. In particular, routine central neck dissection for thyroid cancer increases few times the risk of permanent hypoparathyroidism (20). Hence, the concept of prophylactic central neck dissection for papillary thyroid cancer remains controversial. Despite the fact that an immediate intraoperative autotransplantation is the method of choice during a neck operation for thyroid cancer, the cryopreservation of parathyroid tissue may be rewarding for high-risk patients having more risk for recurrent disease which makes them more likely to undergo revision neck operations in future. Any redo neck surgery, particularly in the central neck, involves an increased risk of permanent hypoparathyroidism approaching to 30% in some series, that can be potentially preventable by reimplantation of cryopreserved parathyroid tissue (3,18). During a redo neck operation an identification and in situ preservation of the functioning of the remaining parathyroid glands is much more difficult as the dissection is undertaken in the scar tissues. In addition, parathyroids left in situ during primary surgery might have been injured as a result of inadequate blood supply preservation. Hence, during a revision central neck dissection, any remaining functional gland inadvertent removal or injury may lead to permanent hypoparathyroidism. In general, it is widely accepted for a redo central neck dissections that any parathyroid gland encountered should be considered to be the last functional parathyroid tissue and should be reimplanted immediately soon after unintentional removal. However, few small pieces of parathyroid tissue can be secured for cryopreservation and possible reimplantation in future if any further neck revisions lead to permanent hypoparathyroidism later on (18).
Cryopreservation techniques
The parathyroid tissue obtained during thyroid surgery is fragmented into 30 to 40 small pieces of 1 mm × 1 mm × 1 mm. The pieces should be kept sterile and put into the chilled saline. Then the supernatant is decanted and usually 8–12 tissue pieces are placed into each vial. Then the containers are deposed in the freezing medium. The use of a standardized cryopreservation method is crucial for preserving graft functionality. The most commonly utilized freezing medium is 80% Roswell Park Memorial Institute (RPMI) 1640 solution (21). The purpose of the freezing procedure is to preserve cellular integrity and function by gradually changing the temperature. Hence, the tubes should be cooled gradually, prior to transfer for a long-term storage. Detailed description of a cooling technique is out of the scope of this publication and can be found in the published literature (22). However, it is important to underline that the most important step for this stage of the process is gradual cooling by −1 °C per minute (22). Once the tubes are cooled, they are put into a liquid nitrogen freezer and stored at temperatures between −170 and −196 °C (22).
Autotransplantation technique
Once the parathyroid tissue pieces are thawed and washed at body temperature few times, they are ready for grafting (23). The non-dominant forearm of the patient is the best site for the parathyroid tissue autotransplantation. Reimplantation is usually undertaken under local anesthesia. Having made surgical incision on the forearm, using a blunt dissection technique, 10–20 individual muscle pockets are created in the brachioradialis muscle and into each of them 1–3 parathyroid graft fragments are reimplanted (23). Caution must be exercised not to cause any hematoma within the intramuscular pockets, which may worsen the vitality of transplants (23).
Functionality assessment and outcomes
Complete independence from calcium and vitamin D3 supplementation is considered the successful transplantation (23). In case the patient requires treatment with calcium slats, with or without vitamin D, with a PTH ratio above 1.5, the transplant is considered to be partially functional (22). However, in case the patient is hypocalcemic in spite of adequate treatment with calcium salts, with or without vitamin D, with a PTH ratio below 1.5, the transplant is considered to be nonfunctional (22). Cryopreserved parathyroid tissue grafts turn out to be functional in less than 70% of patients, whereas the fresh autografts retain functionality in above 90% of patients (21). Cryopreserved parathyroid tissue should be utilized within 18 months, and preferably as soon as possible, as a longer storage reduces markedly the delayed autotransplantation functionality rate (18).
Parathyroid allotransplantation
Nowadays, allotransplantation of cultured parathyroid cells without immunosuppression is an alternative in selected patients to calcium and vitamin D3 supplementation in treatment of permanent hypoparathyroidism. However, it took many years to establish the protocol of cultured parathyroid cells allotransplantation.
From the very beginning it was clear that major histocompatibility complex (MHC) class I and II antigens are expressed on parathyroid tissue limiting ability of direct grafting. In the initial animal studies immunosuppression used after parathyroid tissue allotransplantation reduced the risk of graft rejection to less than 17% compared to 100% rejection rate in animals left without immunosuppression (24). In 1975 Wells et al. published outcomes of parathyroid allograft transplanted within family members who had previously received a kidney transplant and during a 30-month follow-up the graft remained functional (4). In the subsequent years many such transplantations with varying success rate were reported in the literature (3,25). However, taking into consideration the side-effects of life-long immunosuppression this treatment is hardly acceptable for a relatively indolent disease as iatrogenic permanent hypoparathyroidism. Almost a half of parathyroid tissue is composed of non-endocrine and highly immunogenic cells. Human leucocyte antigen (HLA) class I antigens are poorly expressed on parathyroid cells, and are not involved in the induction of the process of host versus graft rejection. Hence, successful and long-term functioning parathyroid allograft is possible when the transplant is free of antigen-presenting cells strongly expressing HLA class II antigens. Thus, allotransplantation of cultured parathyroid cells, but not parathyroids tissue allows for elimination of lymphocytes, macrophages, granulocytes, which represent so called “passenger cells” and endothelial cells presenting HLA class II antigens, responsible to much extent for graft rejection. This innovative method of parathyroid cells culture preparation for allotransplantation without immunosuppression was developed by Woźniewicz et al. (26).
First in the World successful cultured parathyroids cells allotransplantation in humans without immunosuppression for permanent hypoparathyroidism was undertaken by Tołłoczko et al. in Warsaw, Poland in 1990 (27). In 1995 this group reported a series of 18 patients with postoperative parathyroid insufficiency (after thyroid operations) who were treated with cultured, hormonally active, living and ABO the same group type parathyroid cells. Explants had been taken from two operated patients with secondary hyperparathyroidism. Hormonal activity of the transplanted parathyroid cells under the fascia of the brachioradialis muscle had been confirmed by clinical, biochemical tests and the level of PTH in blood serum from the vein of non-grafted arm. Hormonal activity of the graft was variable but lasted up to 14 months (27). In 2007, Nawrot et al. presented encouraging clinical outcomes of parathyroid cells allotransplantation for surgical hypoparathyroidism in a series of 85 patients and described a modified technique of preparing the parathyroid grafts using in vitro breeding (28). The method allowed for obtaining a new parathyroid cell population with a limited expression of immunogenic antigens. As a result of 6 weeks of breeding and freezing, the parathyroid cells markedly decreased their normal HLA class I antigen presenting cells expression and were free of HLA class II positive cells. In this study cells grown using that procedure were allotransplanted without immunosuppression. Sixty patients underwent one allotransplantation, while 25 patients had a repeat grafting. The cellular allografts remained functional for the mean of 6.35±13.08 months. In 64 patients (55.1%), the allografts remained functional for more than 2 months (28). This study protocol served as a basis for development of a clinical protocol of allotransplantation of cultured parathyroid cells which had been finalized in 2015 and this procedure is nowadays reimbursed in Poland by the National Health Fund. In the years 1990–2016 a total number of 316 parathyroid allotransplantations and redo allotransplantations were performed in Warsaw, Poland (personal communication, data not published). Objective improvement considered as increase in serum PTH level and normalization of serum calcium level allowing for decreased in substitution with oral calcium and vitamin D3 was observed in 55.1% of patients. In addition, 34.5% grafts remained functioning within 1–6 months, 10.2% within 6–12 months, 6.2% within 12–24 months, and 1.9% within 24–127 months, respectively. Remarkable improvement in patient quality of life was observed in patients with permanent hypoparathyroidism who had properly functioning graft following allotransplantation, which allowed for majority of them to return to normal professional and social functioning (personal communication, data not published). In case of immediately non-functioning graft or function withdrawal there is still an option of redo allotransplantation as this method is minimally invasive and free of any adverse effects. Hence, in XXI century cultured parathyroid cell allotransplantation without immunosuppression is an alternative in selected patients to substitutive treatment for permanent postoperative hypoparathyroidism. However, it must be emphasized that it is still not widely available. Further research in this area is aimed to develop mechanisms for improvement in graft survival. It was hypothesized that the combination of cultured parathyroid cells and microencapsulation could make parathyroid allotransplantation even a more successful procedure in the management of permanent symptomatic hypoparathyroidism (25,29). Many animal experiments with long-term follow-up have shown that the concept of microencapsulation is effective. However, it remains unclear if the microcapsules prevent immunization itself or rather by the protective effect of tissue separation from the immune response. Nevertheless, it was reported also in humans that using such a combined technique allows for maintaining the functionality of the graft for at least 20 months without requirement of endovenous calcium supplementation (29).
Conclusions
Parathyroid identification and preservation in situ with good vascular supply is the mainstay of safe thyroid surgery. However, if the parathyroid glands are damaged, autotransplantation should be undertaken to preserve their function. Parathyroid transplantation can be considered in three distinct modes of application: (I) fresh parathyroid tissue autotransplantation during thyroidectomy in order to reduce the risk of permanent hypoparathyroidism; (II) cryopreserved parathyroid tissue autotransplantation in patients with permanent hypocalcemia; (III) parathyroid allotransplantation in patients with permanent hypoparathyroidism when cryopreserved parathyroid tissue is not available for grafting. Nowadays, allotransplantation of cultured parathyroid cells without immunosuppression should be taken into consideration in selected patients as an alternative to treatment with calcium and vitamin D3 in the management of permanent hypoparathyroidism.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leem TH, Volpi E, Eisele DW. Non-neural complications of thyroid and parathyroid surgery. In: Randolph GE. editor. Surgery of the thyroid and parathyroid, 2nd ed. Philadelphia: Elsevier Saunders, 2013:446-542.
- Barczyński M, Konturek A, Stopa M, et al. Total thyroidectomy for benign thyroid disease: is it really worthwhile? Ann Surg 2011;254:724-9. [Crossref] [PubMed]
- Saxe A. Parathyroid transplantation: a review. Surgery 1984;95:507-26. [PubMed]
- Wells SA Jr, Gunnells JC, Shelburne JD, et al. Transplantation of the parathyroid glands in man: clinical indications and results. Surgery 1975;78:34-44. [PubMed]
- Olson JA Jr, DeBenedetti MK, Baumann DS, et al. Parathyroid autotransplantation during thyroidectomy. Results of long-term follow-up. Ann Surg 1996;223:472-8. [Crossref] [PubMed]
- Lo CY, Lam KY. Postoperative hypocalcemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: a comparative study. Surgery 1998;124:1081-6; discussion 1086-7. [Crossref] [PubMed]
- Kuhel WI, Carew JF. Parathyroid biopsy to facilitate the preservation of functional parathyroid tissue during thyroidectomy. Head Neck 1999;21:442-6. [Crossref] [PubMed]
- Katz AD. Parathyroid autotransplantation in patients with parathyroid disease and total thyroidectomy. Am J Surg 1981;142:490-3. [Crossref] [PubMed]
- Zedenius J, Wadstrom C, Delbridge L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust N Z J Surg 1999;69:794-7. [Crossref] [PubMed]
- Barczyński M, Cichoń S, Konturek A, et al. Applicability of intraoperative parathyroid hormone assay during total thyroidectomy as a guide for the surgeon to selective parathyroid tissue autotransplantation. World J Surg 2008;32:822-8. [Crossref] [PubMed]
- Paloyan E, Lawrence AM, Paloyan D. Successful autotransplantation of the parathyroid glands during total thyroidectomy for carcinoma. Surg Gynecol Obstet 1977;145:364-8. [PubMed]
- Paloyan E, Lawrence AM, Brooks MH, et al. Total thyroidectomy and parathyroid autotransplantation for radiation associated thyroid cancer. Surgery 1976;80:70-6. [PubMed]
- Lo CY, Lam KY. Parathyroid autotransplantation during thyroidectomy: is frozen section necessary? Arch Surg 1999;134:258-60. [Crossref] [PubMed]
- Lo CY. Parathyroid autotransplantation during thyroidectomy. ANZ J Surg 2002;72:902-7. [Crossref] [PubMed]
- Gauger PG, Reeve TS, Wilkinson M, et al. Routine parathyroid autotransplantation during total thyroidectomy: the influence of technique. Eur J Surg 2000;166:605-9. [Crossref] [PubMed]
- Smith MA, Jarosz H, Hessel P, et al. Parathyroid autotransplantation in total thyroidectomy. Am Surg 1990;56:404-6. [PubMed]
- Lo CY, Luk JM, Tam SC. Applicability of intraoperative parathyroid hormone assay during thyroidectomy. Ann Surg 2002;236:564-9. [Crossref] [PubMed]
- Guerrero MA. Cryopreservation of parathyroid glands. Int J Endocrinol 2010;2010:829540. [PubMed]
- McHenry CR, Stenger DB, Calandro NK. The effect of cryopreservation on parathyroid cell viability and function. Am J Surg 1997;174:481-4. [Crossref] [PubMed]
- White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg 2007;31:895-904. [Crossref] [PubMed]
- Cohen MS, Dilley WG, Wells SA Jr, et al. Long-term functionality of cryopreserved parathyroid autografts: a 13-year prospective analysis. Surgery 2005;138:1033-40; discussion 1040-1. [Crossref] [PubMed]
- Herrera M, Grant C, van Heerden JA, et al. Parathyroid autotransplantation. Arch Surg 1992;127:825-9; discussion 829-30. [Crossref] [PubMed]
- Saxe AW, Spiegel AM, Marx SJ, et al. Deferred parathyroid autografts with cryopreserved tissue after reoperative parathyroid surgery. Arch Surg 1982;117:538-43. [Crossref] [PubMed]
- Wells SA Jr, Burdick JF, Hattler BG, et al. The allografted parathyroid gland: evaluation of function in the immunosuppressed host. Ann Surg 1974;180:805-13. [Crossref] [PubMed]
- Hasse C, Klock G, Schlosser A, et al. Parathyroid allotransplantation without immunosuppression. Lancet 1997;350:1296. [Crossref] [PubMed]
- Woźniewicz B, Migaj M, Giera B, et al. Cell culture preparation of human parathyroid cells for allotransplantation without immunosuppression. Transplant Proc 1996;28:3542-4. [PubMed]
- Tołłoczko T, Sawicki A, Woźniewicz B, et al. Allotransplantation of the parathyroid in patients without immunosuppression. Pol Tyg Lek 1995;50:10-1, 28. [PubMed]
- Nawrot I, Woźniewicz B, Tołłoczko T, et al. Allotransplantation of cultured parathyroid progenitor cells without immunosuppression: clinical results. Transplantation 2007;83:734-40. [Crossref] [PubMed]
- Cabané P, Gac P, Amat J, et al. Allotransplant of microencapsulated parathyroid tissue in severe postsurgical hypoparathyroidism: a case report. Transplant Proc 2009;41:3879-83. [Crossref] [PubMed]


