The current status of robotic transaxillary thyroidectomy in the United States: an experience from two centers
Introduction
Decades after Theodore Kocher mastered the cervical thyroidectomy, surgical innovations in thyroid surgery remained stagnant. Innovations developed during the nascency of laparoscopic abdominal surgery led to the implementation of a variety of minimally invasive techniques in thyroid surgery. Initially, such techniques sought to minimize the length of the cervical incision (1); however, increased tendency to form keloid and hyperplastic scars along with cultural differences in the perception of a neck scar led to the advancement and acceptance of remote access thyroid surgery in East Asian nations (2-4).
Robotic gasless transaxillary thyroid surgery was first introduced by surgeons at Yonsei University in South Korea (5). Researchers at Yonsei University as well as at other tertiary centers in East Asia have demonstrated that robotic thyroidectomy is safe and feasible and provides excellent patient-derived (pain, cosmetic satisfaction) and short term oncologic outcomes (3,6,7). Robotic thyroid surgery garnered much enthusiasm following reports of its success in Asian countries with several centers in the United States adopting the approach (8-11). In 2011, however, after questions arose regarding safety and appropriate attainment of Food and Drug Administration approval for the device, industry support of surgeons performing robotic thyroidectomy procedures was withdrawn (12). In the ensuing years, robotic thyroidectomy procedures have shifted away from high-volume academic centers (13). In this study, trends and outcomes of robotic thyroidectomies performed at two large academic centers are evaluated.
Methods
From June 2009 to May 2016, patients under robotic transaxillary thyroidectomy at Cleveland Clinic (CCF) and the University of Illinois, Chicago (UIC) were maintained in separate IRB-approved prospective databases. Patients with prior neck surgery, nodules greater than 6 cm, obesity, history of Graves’ disease, and suspicion of malignancy with gross extra-thyroidal extension on preoperative ultrasound were excluded from the robotic approach. Pre-operative fiberoptic nasolaryngoscopy was performed on any patient undergoing a completion thyroidectomy or with significant voice symptoms.
After obtaining written informed consent for the procedure, robotic transaxillary thyroidectomy was performed as previously described (11). Briefly, the patient is placed in the supine position with the neck extended. Laterality of approach is determined based on site of largest nodule or malignancy. The ipsilateral arm is placed on an arm board with the elbow flexed at 90-degrees. A 5-cm incision is made along the lateral border of the pectoralis major muscle and a flap is raised anterior to the pectoralis fascia. At the level of the clavicle the plane between the two heads of the sternocleidomastoid muscle (SCM) is entered with routine use of intraoperative ultrasound to help define anatomy and avoid vascular injury. The sternal head of the SCM along with the strap muscles is retracted anteriorly using an elevating retractor thus exposing the central neck and thyroid. At this point the robot is docked using a 30-degree scope, Harmonic scalpel, and Cadiere forceps. A first assistant is available with laparoscopic suction irrigator for counter traction. Thyroidectomy of the ipsilateral lobe is then performed in the usual fashion with identification of the recurrent laryngeal nerve and preservation of parathyroid glands. In those patients undergoing total thyroidectomy, the isthmus is divided and contralateral lobectomy is done in a medial to lateral fashion. The axillary incision is then closed after inspecting for hemostasis. Drains were not routinely used.
Post-operatively, all patients were observed overnight. Serum calcium and PTH levels were checked on post-operative day 1 (POD 1) on all patient who underwent total thyroidectomy. Those patients with diagnosis of well-differentiated thyroid cancer were seen biannually with serum thyroglobulin levels and neck ultrasound. Post-operative radioactive iodine ablation (RAI) for well-differentiated thyroid cancer was performed at the discretion of the treating medical endocrinologist in accordance with American Thyroid Association guidelines. Post-operative fiberoptic nasolaryngoscopy was performed in those patients with persistent voice symptoms 6–12 weeks after surgery.
Results
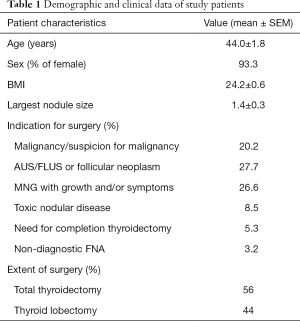
Ninety-four robotic thyroidectomy procedures were performed on 89 patients. Indications for thyroidectomy included biopsy proven malignancy or suspicion for malignancy in 19 patients (20.2%), atypical cells or follicular neoplasm (Bethesda III or IV fine-needle biopsy) in 26 patients (27.7%), multinodular goiter with growth or symptoms in 25 patients (26.6%), toxic nodular disease in 8 patients (8.5%), need for completion thyroidectomy in 5 patients (5.3%), and non-diagnostic fine-needle biopsy in 3 patients (3.2%). Eight patients (8.5%) underwent thyroidectomy for other reasons. Eighty-three (93.3%) patients were female and the remaining 6 (6.7%) were male. Mean age of all patients was 44 years (range, 23–74 years). Average BMI was 24.2 with a range of 16.5 to 41.8. Mean largest nodule size was 1.4 cm (range, 0.1–6 cm). Fifty patients (56%) underwent total thyroidectomy and 39 (44%) lobectomy. Table 1 summarizes the demographic and clinical data of the study patients.
Full table
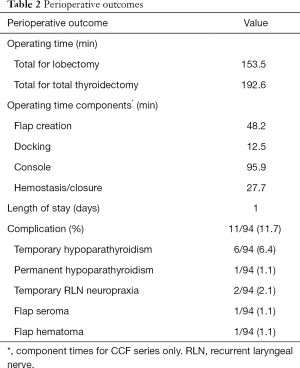
OT was 153.5 minutes for lobectomies and 192.6 minutes for total thyroidectomy. The mean length of stay was 1 day. In those patients who underwent total thyroidectomy, mean POD 1 serum calcium level was 8.5 mg/dL. The complication rate was 11.7% (11 complications in 94 procedures). Temporary recurrent laryngeal neuropraxia in 2 patients (2.3%), permanent hypoparathyroidism in 1 patient (1%), temporary hypoparathyroidism in 6 patients (6.4%), flap seroma in 1 patient (1%), and flap hematoma in 1 patient (1%). There were no temporary or permanent radial, ulnar or median nerve injuries. Table 2 summarizes perioperative data.
Full table
Pathology showed malignancy in 43 patients (48%), including papillary cancer in 22 patients, microscopic papillary cancer in 17 patients and follicular carcinoma in 3 patients. At a mean follow-up of 32.9 months (range, 1–80 months), there were no recurrences or persistent cervical disease identified in cancer patients.
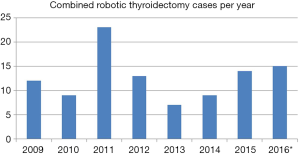
The incidence of robotic thyroidectomy at both institutions reached a peak in 2011 (Figure 1). Since its nadir in 2013, the number of RTs performed has gradually risen. The number of out of state patients increased from 18% to 37% after 2011.
Discussion
To our knowledge, this is one of the largest recent series with 94 procedures reported from the US on robotic transaxillary robotic surgery. The largest series precluding this experience was reported by Kandil et al. in 2012 on 100 procedures (9). This study shows that there is still a demand for remote access thyroid surgery in the US, with 1/3 of the patients traveling significant distances to seek providers. Our results from 2 centers with an advanced endocrine and laparoscopic surgical background, respectively, demonstrate that robotic transaxillary is a safe procedure as long as performed by experienced surgeons in appropriate patients. A recent consensus statement from American Thyroid Association reiterated the findings of this study by accepting the presence of a niche group of patients favoring remote access thyroid surgery over the conventional approach either because of cosmetic or wound healing issues. The panel recommended the wishes of these patients to be respected, as long as the procedure was done by experienced surgeons under strict selection criteria (14).
Despite the popularity and success of robotic thyroid surgery in Asia, the acceptance in the US has been very slow, with a transition of the procedures to low volume centers after withdrawal of industrial support in 2011. In a recent study of 225 patients who underwent robotic thyroid surgery in the US between 2010 and 2011, 93 centers were found to perform robotic procedures, with 89 centers reporting less than 10 cases. The complication rates were higher from lower volume centers (13). In the current study, the morbidity was 11.7%, with no permanent recurrent laryngeal nerve injury, 1% incidence of permanent hypoparathyroidism, and bleeding. These numbers underscore the quality of robotic surgical outcomes in the US when performed by experienced surgeons despite the presence of a much lower volume than in Asia. Our strict patient selection criteria are helpful in maintaining the safety of the procedure. For instance, the procedure is not offered to any patient with evidence of locally advanced thyroid cancer, previous neck surgery or presence of inflammation related to thyroiditis or Grave’s disease.
OT was 2.5 hours for a lobectomy and little over 3 hours for total thyroidectomy in the current series. The OT ranges between 75–250 minutes for a lobectomy and 120–320 minutes for total thyroidectomy in the literature. Our results compare favorably with these numbers. The most challenging part of the procedure is the creation of the flap. In our hands, this was an average of 48 minutes from the Cleveland Clinic series. Our flap times decreased from 50 to 40 minutes in the second, compared to the first part of our experience. The learning curve, according to Chung et al., is 40 cases (15). Our experience is also in accordance with this number. The main part of the learning process is the creation of the flap, as general surgeons do not frequently dissect the transaxillary plane. A tool that helps overcome this learning curve and establish the safety of the procedure is intraoperative ultrasound which helps to identify common carotid artery and internal jugular vein versus the thyroid tissue early on during dissection.
Nerve injuries have been reported related to positioning in robotic transaxillary surgery (16). This is related to undue stretch on the brachial plexus with a certain positioning. The incidence of nerve injuries were zero in the current study due to a modified positioning of the patient’s arm by avoiding more than 90 degrees of extension on the elbow and shoulder joints.
There are no randomized studies in the literature comparing robotic with conventional thyroid surgery in terms of oncologic outcomes. About 1/4 of the patients in the current study had macroscopic papillary thyroid cancer. There were no short-term persistent or recurrent disease seen in these patients.
In conclusion, we report a large experience with robotic transaxillary thyroidectomy from 2 academic centers with this study. Our results show that despite an initial decline in the volume of robotic thyroidectomy procedures after 2011 in the US, there is a consistent small niche of patients preferring remote access over conventional neck surgery. In these patients, good outcomes can be achieved by experienced surgeons if strict patient selection criteria are followed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Data were collected from prospectively-maintained IRB-approved databases (No. 5792).
References
- Miccoli P, Berti P, Conte M, et al. Minimally invasive surgery for thyroid small nodules: preliminary report. J Endocrinol Invest 1999;22:849-51. [Crossref] [PubMed]
- Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg 2007;31:601-6. [Crossref] [PubMed]
- Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. [Crossref] [PubMed]
- Song CM, Yun BR, Ji YB, et al. Long-Term Voice Outcomes After Robotic Thyroidectomy. World J Surg 2016;40:110-6. [Crossref] [PubMed]
- Yoon JH, Park CH, Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech 2006;16:226-31. [Crossref] [PubMed]
- Lee J, Kang SW, Jung JJ, et al. Multicenter study of robotic thyroidectomy: short-term postoperative outcomes and surgeon ergonomic considerations. Ann Surg Oncol 2011;18:2538-47. [Crossref] [PubMed]
- Lee J, Chung WY. Current status of robotic thyroidectomy and neck dissection using a gasless transaxillary approach. Curr Opin Oncol 2012;24:7-15. [Crossref] [PubMed]
- Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope 2011;121:521-6. [Crossref] [PubMed]
- Kandil EH, Noureldine SI, Yao L, et al. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 2012;214:558-64; discussion 564-6. [Crossref] [PubMed]
- Landry CS, Grubbs EG, Morris GS, et al. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery 2011;149:549-55. [Crossref] [PubMed]
- Berber E, Siperstein A. Robotic transaxillary total thyroidectomy using a unilateral approach. Surg Laparosc Endosc Percutan Tech 2011;21:207-10. [Crossref] [PubMed]
- Warning Letter to Intuitive Surgical, Inc. US Department of Health and Human Services, Food and Drug Administration. Issued July 16, 2013. Available online: www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm363260.htm
- Hinson AM, Kandil E, O'Brien S, et al. Trends in Robotic Thyroid Surgery in the United States from 2009 Through 2013. Thyroid 2015;25:919-26. [Crossref] [PubMed]
- Berber E, Bernet V, Fahey TJ 3rd, et al. American Thyroid Association Statement on Remote-Access Thyroid Surgery. Thyroid 2016;26:331-7. [Crossref] [PubMed]
- Lee J, Yun JH, Nam KH, et al. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol 2011;18:226-32. [Crossref] [PubMed]
- Ban EJ, Yoo JY, Kim WW, et al. Surgical complications after robotic thyroidectomy for thyroid carcinoma: a single center experience with 3,000 patients. Surg Endosc 2014;28:2555-63. [Crossref] [PubMed]


