Staged approach to partial breast reconstruction to avoid mastectomy in women with breast cancer
Introduction
The history of use of lateral chest wall flaps for breast reconstruction dates back to 1986. Holmström et al. described Lateral thoracodorsal flap, a random pattern local fascio-cutaneous flap used to assist implant reconstruction after mastectomy for breast cancer (1). The concept of Oncoplastic Breast Surgery has led to emergence of techniques to facilitate partial breast reconstruction (PBR); lateral chest wall perforator flaps (CWPF) being one of them. These flaps extend the indications for breast conservation surgery (BCS) and are associated with minimal procedure related morbidity resulting in quick recovery and excellent aesthetic outcomes.
These are pedicled flaps that could be based on either lateral intercostal artery perforators (LICAP) or branch of lateral thoracic artery (LTA). The other vessels that could be used include anteromedial perforators of intercostal vessels and thoracodorsal artery perforator flap (TDAP) (2). LICAP flaps are based on the lateral cutaneous branch of the posterior intercostal vessels as they course through the costal groove of the ribs. This have been described as a perforator flap that may be used as a free or island flap (3), it has since been used for PBR predominantly for lateral defects after cancer resection (4) and for autologous breast augmentation after massive weight loss (5-7). The LTA is a branch of the 2nd part of the axillary artery with a rich blood supply to the axillary skin, via two to three perforators. LTA can be dissected along the lateral aspect of the pectoralis muscle, running down vertically at right angles to the orientation of the flap (8).
The lateral CWPF is designed on the lateral chest wall by pinching redundant roll of fat with variable extension around the back depending on the tissue needed to fill the defect. The flap is oriented parallel to the skin tension lines with the tip curving up posteriorly parallel to the underlying ribs and following the angiosome description (9). Anteriorly the flap design can be altered to suit the incision required to perform the breast cancer resection, usually a curved line following the lateral inframammary fold to the lateral aspect of the breast. The perforators are preferably marked pre-operatively with a hand-held Doppler with the patient lying down simulating the intra-operative position and the flap design can be moved to ensure the inclusion of more than one perforator. The surgery is performed in lateral position with arm stretched out at 90 degrees in a gutter. All the pre-marked perforators are dissected and none is sacrificed till a dominant pulsatile perforator is found. Once the perforators are dissected, rest of the flap is dissected free and islanded and de-epithelialized. The flap is then inset into the breast by flipping it over on itself or rotating it into the defect.
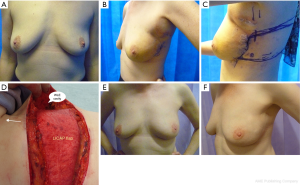
In this article, we are sharing 2-stage approach with lateral thoracic wall perforator flaps for PBR to facilitate BCS in women with breast cancer that borders onto mastectomy (Figures 1A-F). This approach could avoid mastectomy in selected group of women, such as lobular cancers, DCIS, bifocal cancers and post neoadjuvant chemotherapy where pre-operative disease estimation could be challenging; thus extending the indications for BCS. This is a single-center, single surgeon series with prospective data collection.
Methods
This study was performed at Oxford University Hospital, UK. This is a prospective single surgeon series of PBR with lateral CWPF over a 4-year period between 2011–2015.
The data was collected prospectively and updated regularly by collating from histological records, radiological reports for any imaging performed, operative notes for weight of the specimen and type of flap and letters from the oncologists with regards to the treatment received after surgery.
The primary outcomes studied were (I) correlation between pre-operative imaging and pathological tumour size (II) rate of complications after PBR (III) disease characteristics requiring further surgery after an initial attempt at breast conservation and (IV) aesthetic outcomes as assessed by the surgical team and the patients. The study was carried out as a part of routine clinical care with approval to audit the outcomes. The hospital ethical and clinical guidelines were adhered to and patients’ permission was obtained to use their anonymised photographs for educational and publication purposes.
The questionnaire used to assess the patient reported outcomes was Body Image Scale (Appendix) that has been validated for use in women undergoing surgery for breast cancer (Hopwood, Fletcher et al. 2001). The scores were added for all the questions, total could range from 10–40, 10 being the best and 40, worst. The anonymised questionnaires were sent out by a member of the surgical team between 4–6 months after the completion of radiotherapy. Two surgeons (one trainee and one senior surgeon) reviewed preoperative, and 12-month post-op photographs (two views, frontal and oblique) for each patient, the aesthetic outcomes were marked subjectively using Harris scale (10) (poor, fair, good or excellent).
The data were statistically described in terms of mean median and range, or frequencies (number of cases) and percentages when appropriate.
Results
Twenty-three women with diagnosis of DCIS and/or invasive breast cancer were selected for attempt at BCS with 2-stage approach from year 2011–2015. Three patients had extensive disease therefore went on to have mastectomy after wide local excision. Twenty patients underwent successful BCS and had PBR with lateral CWPF within 2–4 weeks after the first operation. The 2-stage approach was adopted in patients where pre-operative disease estimation was difficult to justify one-stage approach. These patients were thought to be a significant risk of needing mastectomy and PBR was best carried after ensuring adequate oncological resection, thus ruling out the need for mastectomy.
All patients have been followed up for median duration of 23 months (17–38 months). The mean age was 49 years (range from 36–62 years); one patient was an active smoker at presentation. All patients were diagnosed pre-operatively with biopsy proven DCIS or invasive breast cancer. The patients were offered the choice of BCS or mastectomy and were counseled with regards to the pros and cons of the two options. The 2-stage approach was adopted in women with high tumour to breast ratio, bordering on to recommendation for mastectomy (expected loss of breast volume of 30% or more) and who expressed the preference for BCS (Figure 1A). All tumours were located in the outer half of the breast, 13 in upper and/or upper-outer quadrant, 4 in outer quadrant and 3 in the lower breast. The bra cup varied from A (1), B (7), C (5) to D (7) cup.
A total of 80 patients underwent PBR in our unit during this time period; three-quarters had one stage approach with cancer resection and PBR performed at the same time. A percentage of 25% patients underwent two-stage approach. The estimated tumour size on pre-operative imaging was significantly higher (P=0.009) in the two-stage group (data published elsewhere) (11).
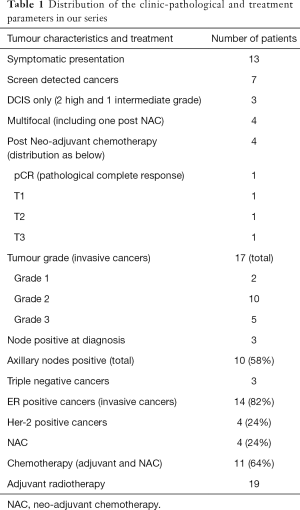
Of 20 patients undergoing staged approach, 13 presented with symptoms and 7 were screen-detected cancers. Seventeen had invasive cancer and three had DCIS only. All patients were assessed pre-operatively by 2-view digital mammogram and ultrasound of the affected breast and axilla. Magnetic resonance imaging scan (MRI) was limited to five patients, the indications being tumour size discrepancy, invasive lobular cancer and patients undergoing neoadjuvant chemotherapy. Out of 17 patients with invasive cancer, 10 (58%) patients had positive lymph nodes; 3 at presentation (proven by ultrasound guided nodal biopsy) and rest after sentinel lymph node biopsy. Four (24%) patients underwent neoadjuvant chemotherapy including the three patients with positive nodes at presentation and fourth patient had triple negative cancer.
The median tumour size judged on pre-op imaging was 43 mm (23–75 mm). These women expressed preference for BCS, therefore wide local excision was performed first and the cavity was maintained patent with normal saline in order to ensure clear margins prior to committing to PBR (Figure 1B). The histopathology was fast-tracked and once margin clearance was ensured, patients were brought back for surgery within 2–4 weeks of initial surgery for PBR to reconstruct the defect.
The median tumour size on final histology was 30 mm (estimated median size of 43 mm on pre-op imaging) in unifocal cancers undergoing primary surgery (total of 16 cases). Two were multifocal (2 or more foci) cancers confined to the same quadrant of the breast and four patients had neoadjuvant chemotherapy with varying degree of response seen on excision. It was observed that out of 13 patients undergoing primary surgery for unifocal cancer, 7 had disease significantly overestimated by pre-op imaging (difference of 10 mm or more) and 3 patients had disease underestimated pre-operatively. In all 7 patients with disease overestimation, the disease extent was estimated to be more than 40 mm on pre-op imaging, whilst the histology revealed disease varying between 18–32 mm; revealing more than 50% overestimation. The median weight of the specimen excised for tumour excision was 79 g (range from 45–200 g). The median size of radial excision margins was 10 mm (range from 2–15 mm).
The complications encountered in this cohort of patients include (I) immediate re-operation for bleeding (one patient) resulting in partial flap loss due to delay in return to theatre leading to volume deficiency and inferior aesthetic outcome; (II) infected seroma (one patient), which was managed conservatively with no significant impact on the further therapy or aesthetic outcome.
Nineteen patients received radiotherapy and 12 out of 17 patients with invasive breast cancer received tumour bed boost. One patient had 30 mm intermediate grade DCIS and decided against adjuvant radiotherapy. Eleven patients (64%) received chemotherapy; 4 as neoadjuvant and 7 as adjuvant therapy (Table 1). Fourteen patients were recommended adjuvant endocrine therapy for ER-positive cancer. Sixteen patients had initial lumpectomy through periareolar scar thus resulting in an additional scar in addition to the scar at flap harvest site, which faded well with radiotherapy.
Full table
The patients are being followed up as per local policy with annual clinical examination and bilateral mammograms. There was no problems reported by the radiologist with regards to mammographic follow-up and patients did not require additional imaging. The median follow-up is 29 months, ranging from 9 to 47 months. There have been no cases of local recurrence to date, two patients presented with distant metastases between 18–24 months after surgery. One patient had triple negative cancer and the other had HER-2 positive heavily node positive disease.
The overall aesthetic outcome (Figures 1E,F) as judged by patients and the surgical team using Harris scale (10) has been good to excellent in 90% patients (18 out of 20 patients). One patient had sub-optimal result due to post-op hematoma and I patient developed breast lymphoedema. No patient has required contralateral symmetrization surgery so far. The patient reported outcome questionnaires with body image scale (12) were sent out to patients within 6 months after completion of in-hospital treatment. Three-quarters responded and 80% reported high satisfaction scores (under 20). The scores received range from 10 to 29, the median score being 16.
Discussion
The lateral CWPF allows PBR in women with small to moderate sized non-ptotic breasts, with a diagnosis of breast cancer and result in excellent aesthetic outcome with minimal morbidity (2,8). These flaps are pivoted at their junction with the vessels therefore have limited mobility making them suitable, essentially, for the lateral breast defects.
LICAP/LTAP flaps are good options for one stage PBR in small to moderate sized non-ptotic breasts with intended tumour excision under 30% of breast volume. In our case series, 2-stage approach was considered in women where breast conservation option was being considered to potentially avoid mastectomy i.e., women with high tumour to breast ratio or the expected loss of breast volume of 30% or more. This approach involves lumpectomy (with axillary procedure, as indicated) and the cavity is filled with saline. The patient is then brought back for flap reconstruction within 2–3 weeks (ideally), once the histology has confirmed negative margins. This approach ensures clear excision margins before embarking on PBR and the procedure is performed without the anxiety of potentially interfering with scarring and reconstruction options should mastectomy be recommended. The potential overestimation of the disease extent on pre-operative imaging, observed in 54% (7 out of 13) of unifocal cancers in our series, makes this approach an attractive and relevant option as it could be difficult to justify mastectomy in these women retrospectively.
The use of various tools such as frozen section for histology and MarginProbe devices for intra-operative margin assessment have been reported in the literature to reduce the need for re-operation (13). The use of intra-operative histology is not efficient due to time constraints and demand on resources (14). There is a significant false negative rate with all the approaches (14,15), which makes the intra-operative analysis questionable for the group of women being selected for staged approach to PBR. Lack of a consistent intra-operative technique to assess for free margins justifies the use of 2-stage approach in selected group of women.
Patient selection is crucial when deciding to adopt two-stage approach. Women with higher tumour to breast size ratio and wishing to conserve their breast are suitable candidates to ensure complete cancer excision before undertaking breast reconstruction. This is overall an effective approach as it avoids unnecessary perforator flap surgery should the patient requires completion mastectomy after an attempt at BCS. This also facilitates disease estimation, thus extending the indications for breast conserving surgery in women, who are otherwise recommended mastectomy based on pre-operative imaging. This is particularly relevant for lobular cancers, DCIS, bifocal cancers and post neoadjuvant chemotherapy, where pre-operative disease estimation could be challenging. There is a definite need for research into pre-operative assessment techniques for better estimation of disease volume. The disadvantages of this approach are (I) potentially more scars as WLE is often performed through peri-areolar approach and (II) logistics of two-operations with limitation on the permissible time period between two operations due to implications of saline absorption.
A low complication rate was observed in our series. Ninety percent patients reported good to excellent aesthetic outcome with high (80%) satisfaction scores. There is better preservation of overall breast shape and proportions, in comparison with total breast reconstruction, due to preserved breast parenchyma and nipple-areola complex. The scar on the lateral chest wall with total incorporation of the flap into the breast helps to define the lateral breast fold (Figure 1F). A high patient satisfaction rate was observed along with maintained breast aesthetics over a period of time despite radiotherapy. A similar study in 2002 (16) reported safety of 2-stage approach with mini LD flap and cosmetically superior results when compared with mastectomy and immediate breast reconstruction in women with breast cancers, thus allowing extension of BCS to women with bigger tumours.
There is published data establishing the oncological safety of oncoplastic procedures (17,18). Our series has a modest median follow-up of 29 months and there was no episode of local recurrence. More than half of cancers were node positive; 4 were Her-2 positive and 3 patients had triple negative cancer suggesting a significant proportion of high risk cases in the case-mix and thus relevance of short term follow-up.
Conclusions
Lateral chest wall redundant fold offer an excellent option for PBR to reconstitute the defect after BCS in carefully selected patients. We recommend two-stage approach in women with high tumour to breast size ratio, in order to avoid mastectomy and ensure successful BCS prior to PBR. This is particularly relevant in women with lobular cancers, DCIS, bifocal cancers and post neoadjuvant chemotherapy, as the pre-operative assessment of tumour extent tends to overestimate disease in a significant proportion of women in routine clinical practice. Our series have shown excellent outcomes with high patient and surgeon satisfaction scores and low complication rates.
Appendix
Body image scale
In this questionnaire you will be asked how you feel about your appearance, and about any changes that may have resulted from your disease or treatment. Please read each item carefully, and draw a circle around the reply which comes closest to the way you have been feeling about yourself, during the last week.
- Have you been feeling self-conscious about your appearance?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you felt less physically attractive as a result of your disease or treatment?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you been dissatisfied with your appearance when dressed?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you been feeling less feminine/masculine as a result of your disease or treatment?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Did you find it difficult to look at yourself naked?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you been feeling less sexually attractive as a result of your disease or treatment?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Did you avoid people because of the way you felt about your appearance?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you been feeling the treatment has left your body less whole?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you felt dissatisfied with your body?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0]. - Have you been dissatisfied with the appearance of your scar?
Not at all [1]; a little bit [2]; quite a bit [3]; very much [4]; not applicable [0].
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The hospital ethical and clinical guidelines were adhered to and patients’ permission was obtained to use their anonymised photographs for educational and publication purposes (ID/number of ethics approval: 4371).
References
- Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg 1986;77:933-43. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Badran HA, El-Helaly MS, Safe I. The lateral intercostal neurovascular free flap. Plast Reconstr Surg 1984;73:17-26. [Crossref] [PubMed]
- Hamdi M, Spano A, Van Landuyt K, et al. The lateral intercostal artery perforators: anatomical study and clinical application in breast surgery. Plast Reconstr Surg 2008;121:389-96. [Crossref] [PubMed]
- De Frene B, Van Landuyt K, Hamdi M, et al. Free DIEAP and SGAP flap breast reconstruction after abdominal/gluteal liposuction. J Plast Reconstr Aesthet Surg 2006;59:1031-6. [Crossref] [PubMed]
- Kwei S, Borud LJ, Lee BT. Mastopexy with autologous augmentation after massive weight loss: the intercostal artery perforator (ICAP) flap. Ann Plast Surg 2006;57:361-5. [Crossref] [PubMed]
- Breuing KH, Colwell AS. Immediate breast tissue expander-implant reconstruction with inferolateral AlloDerm hammock and postoperative radiation: a preliminary report. Eplasty 2009;9:e16. [PubMed]
- McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:686-91. [Crossref] [PubMed]
- Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg 2003;30:331-42. v. [Crossref] [PubMed]
- Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257-61. [Crossref] [PubMed]
- Roy PG. One-stage vs. two-stage approach for partial breast reconstruction with lateral chest wall perforator flaps. Cancer Treat Res 2016;9:56-61.
- Hopwood P, Fletcher I, Lee A, et al. A body image scale for use with cancer patients. Eur J Cancer 2001;37:189-97. [Crossref] [PubMed]
- Gray RJ, Pockaj BA, Garvey E, et al. Intraoperative Margin Management in Breast-Conserving Surgery: A Systematic Review of the Literature. Ann Surg Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Jorns JM, Daignault S, Sabel MS, et al. Is intraoperative frozen section analysis of reexcision specimens of value in preventing reoperation in breast-conserving therapy? Am J Clin Pathol 2014;142:601-8. [Crossref] [PubMed]
- Rivera RJ, Holmes DR, Tafra L. Analysis of the Impact of Intraoperative Margin Assessment with Adjunctive Use of MarginProbe versus Standard of Care on Tissue Volume Removed. Int J Surg Oncol 2012;2012:868623. [Crossref] [PubMed]
- Dixon JM, Venizelos B, Chan P. Latissimus dorsi mini-flap: a technique for extending breast conservation. Breast 2002;11:58-65. [Crossref] [PubMed]
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012;38:395-8. [Crossref] [PubMed]
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: A matched-cohort analysis. Eur J Surg Oncol 2016;42:71-7. [Crossref] [PubMed]


