A rare presentation of malignant phyllodes tumor with bloody nipple discharge—report of a case
Introduction
Phyllodes tumor, first characterized by Johannes Muller in 1838, is an uncommon fibroepithelial tumor of the breast (1). Compared with female breast carcinomas, phyllodes tumors constitute 0.3–0.5% of breast tumors (2) and it typically appears in women’s 30s or 40s. There are suggestions that this figure may be significantly higher in Asian women (3). This tumor is characterized by its structural similarity to the fibroadenoma with low mitotic activity, a biphasic proliferation of stromal and epithelial component. Here we reported a case of a 22-year-old woman who presented with the clinical symptoms of palpable tumor and nipple discharge, preoperatively suggestive of an intraductal papilloma; however, the microscopic examination showed a malignant phyllodes tumor with intraductal growth.
Case presentation
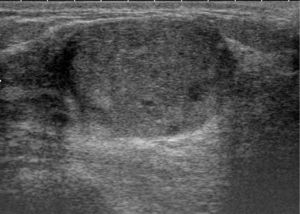
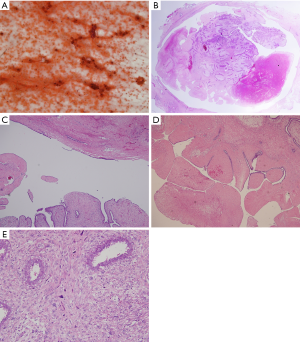
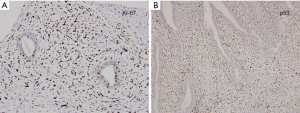
A 22-year-old woman with no known risk factors for breast cancer was presented to our hospital because of right breast tumor for 5 years and nipple discharge developed in one month before this admission. Physical examination confirmed a 3-cm well-defined tumor located at 7 o’clock near the areola and there was brown color discharge from a single duct opening while squeezing the tumor. The ultrasound disclosed a 3.5-cm well defined tumor with hypoechoic pattern in her breast. Cystic regions within the tumor and ductal extension of the tumor were also found (Figure 1). The cytology of nipple discharge showed hemosiderin-laden macrophages, degenerative ductal epithelium and negative result for malignancy (Figure 2A). Under the preoperative diagnosis of intraductal papilloma or fibroadenoma, she received excisional biopsy. The specimen showed a well-defined, white, firm tumor grossly. Histologically, sections showed intraductal circumscribed tumor with pushing border and was composed of spindle cell stroma and benign glandular elements (Figure 2B). Focally markedly hypercellular stroma with markedly cellular pleomorphism, tumor giant cells and atypical mitotic feature are noted (Figure 2C,D). The highest mitotic count is about 12 mitotic figures per 10 HPF (Figure 2E). Immunohistochemical tests showed 50% positivity for Ki-67 and positive expression for p53 (Figure 3). The histologic diagnosis was malignant phyllodes tumor with intraductal growth. Thus, she received skin-sparing total mastectomy and immediately reconstruction with silicone gel implant. No axillary lymph node dissection was performed. The postoperative course was uneventful.
Discussion
Phyllodes tumor presented with nipple discharge as the main symptom is an infrequent mode of presentation. Norris and Taylor had an analysis of 94 phyllodes tumor (4) and they found only one patient presented with nipple discharge. There are four other reports of phyllodes tumor presented with nipple discharge in the literature (5-8). All of these cases were benign or borderline phyllodes tumors and three of them were under the age of 20.
A case of phyllodes tumor with nipple discharge and intraductal growth was reported in 2007 by Lian et al. (7) in which the tumor measured 16 mm × 14 mm × 9 mm. This report shares some similarities with our case, although our case was malignant and larger. To our knowledge, this is the first case of malignant phyllodes tumor presented with symptoms of palpable tumor and bloody nipple discharge.
The etiologies of nipple discharge have been divided into non-neoplastic and neoplastic in Sakorafas’s review article (9). The non-neoplastic causes are galactorrhea, physiologic discharge, pregnancy, periductal mastitis, mammary duct ectasia, or fibrocystic changes. The neoplastic causes would be attributed to intraductal papilloma, papillomatosis, or underlying malignancy. For phyllodes tumor with nipple discharge, exaggerate periductal stromal cell proliferation could be a candidate. The causes of nipple discharge in phyllodes tumor may be different. For example, bloody nipple discharge is probably due to spontaneous infarction of the tumor (5); on the other hand, clear nipple discharge may result from accumulated secretions within the major breast ducts as a consequence of blockage of the ducts by the intraductal phyllodal growth (7). In our case, the cytology exam of nipple discharge revealed hemosiderin-laden macrophages which hint there was bleeding episodes before and that it may be caused by ductal invasion of the tumor.
Phyllodes tumors with intracystic growth had been reported (10,11). Findings of intracystic phyllodes tumor in ultrasound and pathology are similar to those of intraductal phyllodes tumor. But symptoms of nipple discharge are usually absent in intracystic phyllodes tumor. An intraductal growth pattern of phyllodes tumor may have implications for both diagnosis and management. The clinical symptoms of palpable tumor with nipple discharge were highly suggestive of a ductal lesion. In our case, a 22-year-old woman, the tumor was observed for 5 years which suggested fibroadenoma. But the latter developed symptoms of nipple discharge and led the diagnosis to be malignancy or a ductal tumor. The evaluation tests for patients with nipple discharge in our hospital include ultrasound and cytology. Mammography is also indicated depending on patient’s age. We do not perform galactography or magnetic resonance image (MRI) routinely. We think that breast ultrasound is superior to the galactography for it is non-invasive. In addition, ductal systems more than 0.5 mm and relationship of involved duct system with single or multiple intraductal lesions can be clearly demonstrated by breast ultrasound. MRI may also be considered due to its dynamic enhancement function for demonstrating relationship between the tumor and duct systems. However, patients in Taiwan need to pay an extra fee for breast MRI because it is not covered by Taiwan’s National Health Insurance.
For management of nipple discharge without palpable tumor, microdochectomy was our first choice to be performed for diagnosis and treatment. As for phyllodes tumor with nipple discharge, microdochectomy and wide local excision with adequate margins of surrounding normal breast tissues are more proper procedures. Injection of methylene blue during microdochectomy probably can demonstrate the relationship between tumor and the affected ducts. Excisional biopsy was done initially for our case because of clinically suspicious of fibroadenoma. Unfortunately, the histological exam revealed a malignant phyllodes tumor. Due to symptom of bloody nipple discharge and central located tumor, we performed skin-sparing total mastectomy with immediately reconstruction for her. No axillary lymph node dissection was needed.
Immunohistochemical study was applied to this tumor. MIB1 (Ki-67) was 50% positivity for the tumor and p53 protein was positive. Dacic et al. demonstrated a correlation between MIB1 (Ki-67) positivity and histologic high-grade tumors (12). Examination of the stromal cells is important in showing the characteristics of phyllodes tumors, because only the stromal components metastasize. Kim et al. (13) investigated p53 expression of stromal cells in phyllodes tumors and reported that none of the eight benign phyllodes tumors expressed p53 whereas six of the seven malignant phyllodes tumors expressed this protein. The expression of p53 may be a possible indicator of malignant phyllodes tumor. Based on these findings, our data is compatible with these studies.
Preoperative diagnostic accuracy of phyllodes tumor allows correct surgical treatment, avoiding the pitfalls of reoperation because of inadequate excision, or surgical overtreatment. Jacklin et al. propose the Paddington Clinicopathologic Suspicion Score (14) to assist in the selection of patients for core biopsy. And they concluded that when clinico-radiological features of phyllodes tumour are present, core biopsy is preferred owing to the high rates of false negative/indeterminate findings for fine needle aspiration cytology. As we know, fibroadenomas and phyllodes tumors have similar cytologic features.
After resection of a phyllodes tumor, regular follow-up is mandatory. A clinical breast examination and new baseline imaging should be arranged at 6 months postoperatively.
Acknowledgements
Special thanks to Miss Jeng-Ya Hsin for English correction.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Calhoun KE, Lawton TJ, Kim JN, et al. Phyllodes tumors. In: Harris JR, Lippman ME, Morrow M, et al., eds. Diseases of the Breast, 4th edition. Philadelphia: Lippincott Williams and Wilkins, 2010: 781-92.
- Reinfuss M, Mituś J, Duda K, et al. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996;77:910-6. [Crossref] [PubMed]
- Tan PH, Jayabaskar T, Chuah KL, et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol 2005;123:529-40. [Crossref] [PubMed]
- Norris HJ, Taylor HB. Relationship of histologic features to behavior of cystosarcoma phyllodes. Analysis of ninety-four cases. Cancer 1967;20:2090-9. [Crossref] [PubMed]
- Tagaya N, Kodaira H, Kogure H, et al. A Case of Phyllodes Tumor with Bloody Nipple Discharge in Juvenile Patient. Breast Cancer 1999;6:207-210. [Crossref] [PubMed]
- Martino A, Zamparelli M, Santinelli A, et al. Unusual clinical presentation of a rare case of phyllodes tumor of the breast in an adolescent girl. J Pediatr Surg 2001;36:941-3. [Crossref] [PubMed]
- Lian D, Cheah E, Tan PH, et al. Phyllodes tumour with intraductal growth: a rare cause of nipple discharge. Histopathology 2007;50:666-9. [Crossref] [PubMed]
- Pistolese CA, Tanga I, Cossu E, et al. A phyllodes tumor in a child. J Pediatr Adolesc Gynecol 2009;22:e21-4. [Crossref] [PubMed]
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001;27:275-82. [Crossref] [PubMed]
- Santosh KV, Sumana BS. Benign intracystic phyllodes tumor of breast. Indian J Pathol Microbiol 2010;53:385-6. [Crossref] [PubMed]
- Horiguchi J, Iino Y, Aiba S, et al. Phyllodes tumor showing intracystic growth: a case report. Jpn J Clin Oncol 1998;28:705-8. [Crossref] [PubMed]
- Dacic S, Kounelis S, Kouri E, et al. Immunohistochemical profile of cystosarcoma phyllodes of the breast: a study of 23 cases. Breast J 2002;8:376-81. [Crossref] [PubMed]
- Kim CJ, Kim WH. Patterns of p53 expression in phyllodes tumors of the breast--an immunohistochemical study. J Korean Med Sci 1993;8:325-8. [Crossref] [PubMed]
- Jacklin RK, Ridgway PF, Ziprin P, et al. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol 2006;59:454-9. [Crossref] [PubMed]


