Preoperative psychosocial characteristics may predict body image and sexuality two years after risk-reducing mastectomy: a prospective study
Introduction
Risk reducing mastectomy (RRM) is an established preventive alternative for women at high risk for breast cancer, considered to give a reduction in breast cancer by more than 90% (1,2). Women with a family history of breast cancer and/or identified BRCA mutations may opt for bilateral risk-reducing mastectomy (BRRM), whereas patients with a personal history of breast cancer may consider contralateral risk-reducing mastectomy (CRRM). RRM is irrevocable and includes issues of psychological distress, negative impact on body image, sexual functioning and potential surgical complications (3). The clinical course for patients undergoing contralateral RRM, evaluated in median 4 years later, showed that 60% required at least one reoperation (4).
Information about what to expect after RRM is of outmost importance for women facing taking a decision to undergo RRM. Relatively few prospective studies on psychosocial consequences of undergoing RRM with long-term follow-up have been published (5). In one study from the Netherlands, the course of psychological distress and body image at long-term follow-up (6–9 years) after prophylactic mastectomy and breast reconstruction were explored in 36 women at risk for hereditary breast cancer (6). The results revealed decreased breast cancer worry, but persistent body image problems. Active coping and seeking social support were predictive of lower levels of body image problems at the long-term follow up. In another prospective study from the Netherlands of 48 healthy BRCA1/2 mutation carriers, body image decreased 6 months after BRRM (7). Two years postoperatively, both body image and satisfaction with sexual relationship tended to be lower as compared to baseline. In addition, 37% of the women reported at this assessment point that their breasts felt unpleasant, 29% that they were not satisfied with the appearance of their breasts, and 21% that they felt embarrassed for their naked body. Most of the body image issues remained, however, unchanged in 30% of the women. High preoperative cancer distress predicted negative body image at follow-up. In a US study, 621 unilateral breast cancer patients with a family history of breast cancer who underwent CRRM between 1960 and 1993 were surveyed regarding HRQoL and satisfaction with CRRM at two time points, 10 and 20 years, after CRRM (8). The results revealed that most women reported stable long-term satisfaction with the CRRM. Women who had breast reconstruction and required reoperations reported, however, lower satisfaction.
Finally, a recent systematic review of the PROMs after bilateral risk-reducing mastectomy that included 22 studies reported that patients after BRRM are generally satisfied with the outcomes, body image and psychosocial well-being, whilst sexual well-being and somatosensory function are negatively affected (9). Of note, the authors hypothesized that preoperative distress could be a predictor of quality of life and body image, and that more longitudinal studies with validated instruments are needed to observe changes in PROMs over time.
Since 1992, PM has been considered at the Karolinska University Hospital for women at a high risk of developing breast cancer (10). A collaborative group, consisting of geneticists, oncologists, breast surgeons, plastic surgeons, nurses and a psychologist, was established at the Karolinska University Hospital in 1996 in order to meet the increasing interest in RRM in women at high hereditary risk. A prospective questionnaire study of the expectations on RRM, satisfaction with the result of RRM, emotional reactions, body image, sexuality and HRQoL were conducted, including women who underwent RRM between 1997 and 2010, and the results have been published in a number of papers (4,10-15). In conclusion, the results showed that unaffected women, opting for bilateral RRM, reported similar HRQoL levels as a normative population, whereas patients opting for contralateral RRM were comparable to breast cancer patients (10). The prospective one-year follow-up revealed problems with body image and sexuality after BRRM, but no increase in emotional distress or in HRQoL (11). A majority reported high overall satisfaction with the cosmetic results six months and one year after BRRM, corresponding to their expectations (12). At 2-years follow up, a majority reported pain and discomfort in the breasts, as well as reduced sexual sensations (13,14). Finally, the prospective 2-years follow-up of women with breast cancer who had CRRM showed no adverse effects on HRQoL, emotional distress or sexuality, but some aspects of body image were affected negatively (15).
Considering the consistent findings of problems with body image and sexuality long-term after RRM, it is important to be able to identify women at risk for these problems as early as before the operation in order to provide support. The aim of the present study was to investigate the association between baseline HRQoL and emotional distress (anxiety and depression) and body image and sexuality 2 years after RRM surgery.
Methods
All women opting for RRM due to hereditary increased risk for breast cancer at Karolinska University Hospital during 1998–2010 were offered a consultation with a medical psychologist (YB) as part of the established preoperative assessment. From the start of the study, women with a modest or highly increased risk were offered PM, but from 1999, only women with an estimated risk of ≥40% based on family history or genetic testing were offered to undergo RRM. The women were invited to participate in the questionnaire study as described elsewhere at the end of the consultation with YB (10,11). The patients were given a set of questionnaires to be completed at baseline, i.e., preoperatively and a prepaid envelope. Two years after the date of RRM, the patients were sent a postoperative set of questionnaires, and a prepaid return envelope. A reminder including a new set of questionnaires was sent to those who did not respond within two weeks.
Preoperative assessment for patients who opted for RRM included a mandatory oncogenetic counselling by clinical oncologists with breast cancer risk estimation, and with BRCA1/BRCA2 genes screening in most cases. Surgical techniques and breast reconstruction have been described in detail previously and were not changed during the study period (3,4). In brief, a subcutaneous skin-sparing mastectomy with immediate implant-based breast reconstruction was performed, using expandable or permanent implants. Nipple re-transplanting and areola tattooing were done in most patients under local anaesthesia at a later stage.
Questionnaires
The Medical Outcomes Study 36-Item Short Form (SF-36) is a well-known generic instrument used to assess HRQoL. Its concepts are not specific to age, disease or treatment group (16). It includes 36 items, defining eight HRQoL domains: physical functioning, role limitations due to physical problems, bodily pain, social functioning, general mental health, role limitations due to emotional problems, vitality, and general health perception (17). Each of eight domains ranges from 0 to 100, where high figures represent higher level of functioning and HRQoL. The Swedish version has been validated (17).
The hospital anxiety and depression scale (HADS) is one of the most widely used instruments for the assessment of anxiety and depression in somatically ill patients (18). The 14 items of HAD constitutes two subscales, the anxiety subscale (7 items) and the depression subscale (7 items). Higher scores indicate higher levels of anxiety and depression (range 0–21 for each of the subscales). The Swedish version has been validated against diary recordings in a sample of breast cancer patients (19).
The sexual activity questionnaire (SAQ) was developed to assess sexual functioning and changes in sexual activity (20). The 10 items of SAQ constitute three variables: pleasure (6 items, summated score from 0 to 18), discomfort (2 items, summated score from 0 to 6) and habit (1 item, scored from 0 to 3). Higher scores indicate more pleasure and more discomfort, respectively, in the habit section score <1 indicates less frequent than usual. The Swedish translation, performed by a multi-disciplinary group, has not been formally validated, but utilized in a number of our breast cancer studies over the last decade.
The body image scale (BIS) evaluates the impacts of surgery on patients’ self-consciousness, physical and sexual attractiveness, femininity, satisfaction with body and scars, body integrity, and avoidance behaviour (21). The 10-item scale is scored from 0 (not at all) to 3 (very much). The sum of the BIS-items gives a summated score (range, 0-30), where higher score represent more problems/negative changes. The scale, designed to be used postoperatively, were sent to the patients 2 years after RRM. The translation to Swedish was performed by the same multi-disciplinary group as the translation of the SAQ, and has not undergone formal validation.
Statistical analysis
All single items in the SF-36 were transformed into the eight subscales with scores ranging 0-100 according to the scoring manual (17). High figures represent higher level of functioning and HRQoL.
The HADS anxiety and depression subscales were analysed as two continuous variables each ranging from 0 to 21 according to the original publication (18).
For the BIS presentation, all items were combined into a BIS summated score (range, 0–30), but the frequencies for individual items were also presented.
The SAQ subscales were analysed according to the original publication, i.e. pleasure (range, 0–18), discomfort (range, 0–6), and habit (range, 0–3) (20).
All patients were grouped according to having undergone BRRM or CRRM. Associations between SF-36, HADS, BIS, SAQ were analysed using linear regression models unadjusted, and adjusted for age at RRM as continuous variable. No notable changes were found when adjusted for calendar year (data not presented).
All associations in the regression models were calculated only for the patients with both pre- and postoperative data available. The results are presented as slopes (beta-coefficients) and 95% confidence intervals. Reported P values refer to Wald tests. In the linear regression, only preoperative and postoperative
The level of statistical significance was set to 0.05% and 95% confidence intervals (95% CIs) were presented accordingly.
STATA/SE (Version 13.1; StataCorp, TX, USA) was used for all statistical analyses.
Results
During the study period, 405 women were referred to the psychologist (YB). Among them, 276 patients underwent bilateral (BRRM, n=179, 65%) or unilateral (i.e., contralateral, CRRM, n=97, 35%) RRM. The remaining 119 patients did not go through RRM during the study period.
In total, 253 patients (92%) consented to participate in the study between January 1998 and December 2010 by sending back a completed questionnaire at baseline and/or postoperatively. Twenty-three women (8%) did not return the questionnaires and were thus considered as non-participants. Response rate at baseline was 222/253 (88%), including 148 BRRM (67%) and 74 CRRM (33%) patients. Corresponding figures at the two-years assessment were 179 (71%), 115 BRRM (64%) and 64 CRRM (36%).
The mean age at the baseline assessment was 44 years (range, 25–75 years). Women in the BRRM group appeared to be somewhat younger than the CRRM patients, 43 years (95% CI: 41–44 years) vs. 46 years (95% CI: 44–48 years), although not statistically significant (P=0.067).
Baseline HRQoL and body image two years after RRM
In the BRRM group (healthy women), significant negative relationships between SF-36 subscales at baseline and the BIS score 2 years after RRM were found throughout all the SF-36 domains, both in the unadjusted and age-adjusted analyses, Table 1, left.
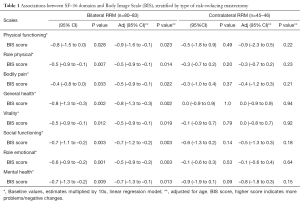
Full table
In the CRRM group (breast cancer patients) no correlations between SF-36 subscales and the BIS score appeared in any of the domains, Table 1, right.
Baseline HRQoL and sexuality two years after RRM
In both the unadjusted and the age-adjusted analyses, the SAQ variables in the BRRM group were negatively associated with the following SF-36 subscales: General Health (habit), Vitality (pleasure, discomfort), Social functioning (pleasure, discomfort), Role Emotional (habit) and Mental Health (pleasure, discomfort), Table 2, left. No associations were found between the SAQ variables and the SF-36 subscales Physical functioning, Role functioning and Bodily Pain.

Full table
For patients undergoing CRRM, none of the SAQ variables was associated with the baseline SF-36 subscales in the regression analyses, Table 2, right.
Baseline anxiety/depression and body image two years after RRM
No association between the HADS anxiety score and the BIS score was revealed in the BRRM group. An increase by 1 unit on the HADS depression scale was statistically significantly associated with an increase in the BIS score, and remained significant in the age-adjusted analysis, Table 3, left.
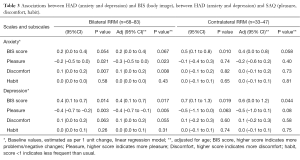
Full table
Preoperative level of HADS anxiety in the CRRM group was positively associated with the BIS score in the unadjusted analysis. This association was, however, no longer statistically significant when adjusted for age, Table 3, right. Also in this group an increase in the HADS depression score was associated with negative changes in the BIS score in both analyses.
Baseline anxiety/depression and sexuality two years after RRM
In the BRRM group, the baseline HADS anxiety score was negatively associated with a decrease in pleasure and an increase in discomfort in both the unadjusted and the adjusted analyses. The HADS depression level revealed an association with decrease in pleasure.
No associations between the baseline HADS and the SAQ subscales were identified in the CRRM group (Table 3, right).
Discussion
The results revealed that preoperative HRQoL and emotional distress were associated with body image and sexual problems 2 years after the procedure in high-risk women without breast cancer undergoing BRRM. Interestingly, we did not found similar associations for the women with breast cancer who underwent CRRM. In this group, the only associations found were between the baseline anxiety (univariate analysis) and depression scores and body image 2 years after CRRM. As body image and sexuality have previously been reported as persisting problems in women undergoing BRRM, the results indicate an opportunity to identify, already before surgery, women with hereditary risk without breast cancer who are vulnerable for these problems by using the SF-36 and HAD questionnaires. These questionnaires appear, however, to be less efficient in identifying vulnerable women with breast cancer undergoing CRRM.
The concept “Body image” has been described as: “a multifaceted construct, defined as the mental representation of one’s body, thoughts and feelings about one’s physical appearance, attractiveness and competence, as well as one’s perceived state of overall health, wholeness, functioning and sexuality (22). The concepts of body image and sexual functioning are thus interrelated. We chose, however, to investigate body image and sexual functioning separately, as both of them have been shown to be negatively affected. A study of sexual functioning in breast cancer survivors experiencing body image disturbance found no correlation between body image, using the BIS, and sexual functioning, further supporting our approach of separating the concepts (23).
Previous studies have shown associations between HRQoL, and body image and sexual functioning in breast cancer patients. HRQoL and body image were found to be negatively associated in a study of 150 women with breast cancer after adjuvant radiotherapy and/or chemotherapy (22). Bredart and co-workers (24) found, in 378 women with early stage breast cancer about six months to five years after radiotherapy, presence of lower HRQoL scores to be associated with a decreased frequency of sexual activity, decreased sexual pleasure and greater sexual discomfort, assessed by SAQ. A prospective study of 223 women with early stage breast cancer investigated the associations between anxiety, depressive symptoms and fatigue, and quality of sexual life, sexual functioning and sexual enjoyment at six and twelve months after surgical treatment (25). No associations with clinical factors were found, but “trait anxiety” and “extraversion” showed an association with sexual life and sexual functioning 6 months after breast cancer surgery. No associations were found for depression. In a German study, however, including 98 women with breast cancer undergoing adjuvant treatment, depressive symptoms predicted the women’s “body image–self-acceptance” (26). In a questionnaire study of 60 women about 52 months after prophylactic mastectomy, HRQoL scores were negatively associated with cancer-related distress, body image difficulties, and psychological distress (27). Thus, previous research has established associations between HRQoL and emotional distress, and body image and sexual functioning. We therefore hypothesized negative associations between the HRQoL and emotional distress variables, and body image and sexual functioning, except for the SAQ “Pleasure scale”, where a positive association was expected. Our results for the BRRM group indicated associations between all HRQoL variables and body image, but only for some sexuality functioning variables, partly confirming our hypotheses. Surprisingly, we did not find similar associations for the CRRM group, contrary to previous international findings. One reason for this might be that most previous studies have been cohort studies, exploring associations at one time point. Thus, there is a higher possibility to find associations between related psychosocial variables. In our study, the baseline data on HRQoL and emotional distress were collected at least two years before the body image and sexuality functioning data. Another reason is the low number of patients in the CRRM group being sexually active and responding to SAQ, only 32 women (33%) as compared to 68 (38%) in the BRRM group. The women in the CRRM group appeared to be slightly older than the women in the BRRM group, possibly partly explaining the difference in sexual activity. Brédart and co-workers (24) found an association between older age and lower frequency of sexual activity in women with breast cancer.
We chose to investigate a non-cancer specific measure of HRQoL, the SF-36 and an instrument for assessment of emotional reactions, the HADS for use as a screening tool for later problems with body image and sexuality, assessed by the SAQ and the BIS. The BIS was not administered before RRM, thus it was not possible to investigate the associations between the baseline BIS and the assessment two years later. The SAQ was not chosen as it was assumed that there would be an association between the two assessments as they are dependent. In addition, some women might be hesitant to complete the SAQ due to the intimate content. A third reason for the choice of investigating HRQoL and emotional reactions was that support in order to improve HRQoL and relieve emotional problems were considered to be available to a greater extent in the clinic than interventions to improve sexual functioning.
Some weaknesses of the present study include the limited number of patients who returned postoperative questionnaires, particularly in the CRRM group. The study was a part of the clinical routine for women with high risk for breast cancer and it was voluntary to participate by completing questionnaires. Thus, it was expected that some women would reject participation in the study.
We did not investigate the perception of the couple relationship, which has been demonstrated to be the most important variable for sexual functioning (7,24,26). It is plausible that satisfaction with the relationship at baseline may be associated with sexual functioning two years after RMM. There is, however, a risk that the relationship with partner will change after RRM as a result of body image problems, which is not possible to foresee before RRM (28).
The strengths of this study lie in its prospective design and long follow-up with validated questionnaires. The impact of bilateral (i.e., asymptomatic women) and contralateral (i.e., unilateral cancer patients) risk-reducing mastectomy has been assessed separately. During the study period, all patients were treated using the same clinical routines, which was additionally checked in the multivariable analysis adjusted for “calendar year” with no marked changes in the regression model.
Conclusions
The current study suggests that baseline HRQoL and psychological distress, assessed by SF-36 and the HADS, may be useful to identify women at risk for long-term body image and sexual problems following bilateral risk-reducing mastectomy. The clinical significance of the study could be that the results of preoperative assessment may predict the postoperative patient-reported outcomes.
Acknowledgements
Funding: This study was funded by the Swedish Cancer Society (Cancerfonden) (Grant number 090651).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee at Karolinska Institutet (Dnr 2005/685-31).
References
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol 2005;23:4275-86. [Crossref] [PubMed]
- Arver B, Isaksson K, Atterhem H, et al. Bilateral prophylactic mastectomy in Swedish women at high risk of breast cancer: a national survey. Ann Surg 2011;253:1147-54. [Crossref] [PubMed]
- Unukovych D, Sandelin K, Wickman M, et al. Breast reconstruction in patients with personal and family history of breast cancer undergoing contralateral prophylactic mastectomy, a 10-year experience. Acta Oncol 2012;51:934-41. [Crossref] [PubMed]
- Tracy MS, Rosenberg SM, Dominici L, et al. Contralateral prophylactic mastectomy in women with breast cancer: trends, predictors, and areas for future research. Breast Cancer Res Treat 2013;140:447-52. [Crossref] [PubMed]
- den Heijer M, Seynaeve C, Timman R, et al. Body image and psychological distress after prophylactic mastectomy and breast reconstruction in genetically predisposed women: a prospective long-term follow-up study. Eur J Cancer 2012;48:1263-8. [Crossref] [PubMed]
- Gopie JP, Mureau MA, Seynaeve C, et al. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in healthy women at risk for hereditary breast cancer. Fam Cancer 2013;12:479-87. [Crossref] [PubMed]
- Boughey JC, Hoskin TL, Hartmann LC, et al. Impact of reconstruction and reoperation on long-term patient-reported satisfaction after contralateral prophylactic mastectomy. Ann Surg Oncol 2015;22:401-8. [Crossref] [PubMed]
- Razdan SN, Patel V, Jewell S, et al. Quality of life among patients after bilateral prophylactic mastectomy: a systematic review of patient-reported outcomes. Qual Life Res 2016;25:1409-21. [Crossref] [PubMed]
- Brandberg Y, Arver B, Lindblom A, et al. Preoperative psychological reactions and quality of life among women with an increased risk of breast cancer who are considering a prophylactic mastectomy. Eur J Cancer 2004;40:365-74. [Crossref] [PubMed]
- Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol 2008;26:3943-9. [Crossref] [PubMed]
- Brandberg Y, Arver B, Johansson H, et al. Less correspondence between expectations before and cosmetic results after risk-reducing mastectomy in women who are mutation carriers: a prospective study. Eur J Surg Oncol 2012;38:38-43. [Crossref] [PubMed]
- Gahm J, Wickman M, Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer--prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast 2010;19:462-9. [Crossref] [PubMed]
- Gahm J, Hansson P, Brandberg Y, et al. Breast sensibility after bilateral risk-reducing mastectomy and immediate breast reconstruction: a prospective study. J Plast Reconstr Aesthet Surg 2013;66:1521-7. [Crossref] [PubMed]
- Unukovych D, Sandelin K, Liljegren A, et al. Contralateral prophylactic mastectomy in breast cancer patients with a family history: a prospective 2-years follow-up study of health related quality of life, sexuality and body image. Eur J Cancer 2012;48:3150-6. [Crossref] [PubMed]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [Crossref] [PubMed]
- Sullivan M, Karlsson J, Ware JE Jr. The Swedish SF-36 Health Survey--I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995;41:1349-58. [Crossref] [PubMed]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [Crossref] [PubMed]
- Arving C, Glimelius B, Brandberg Y. Four weeks of daily assessments of anxiety, depression and activity compared to a point assessment with the Hospital Anxiety and Depression Scale. Qual Life Res 2008;17:95-104. [Crossref] [PubMed]
- Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: a measure of women's sexual functioning. Qual Life Res 1996;5:81-90. [Crossref] [PubMed]
- Hopwood P, Lee A, Shenton A, et al. Clinical follow-up after bilateral risk reducing ('prophylactic') mastectomy: mental health and body image outcomes. Psychooncology 2000;9:462-72. [Crossref] [PubMed]
- Boquiren VM, Esplen MJ, Wong J, et al. Exploring the influence of gender-role socialization and objectified body consciousness on body image disturbance in breast cancer survivors. Psychooncology 2013;22:2177-85. [PubMed]
- Boquiren VM, Esplen MJ, Wong J, et al. Sexual functioning in breast cancer survivors experiencing body image disturbance. Psychooncology 2016;25:66-76. [Crossref] [PubMed]
- Brédart A, Dolbeault S, Savignoni A, et al. Prevalence and associated factors of sexual problems after early-stage breast cancer treatment: results of a French exploratory survey. Psychooncology 2011;20:841-50. [Crossref] [PubMed]
- Den Oudsten BL, Van Heck GL, Van der Steeg AF, et al. Predictors of depressive symptoms 12 months after surgical treatment of early-stage breast cancer. Psychooncology 2009;18:1230-7. [Crossref] [PubMed]
- Zimmermann T, Scott JL, Heinrichs N. Individual and dyadic predictors of body image in women with breast cancer. Psychooncology 2010;19:1061-8. [Crossref] [PubMed]
- Metcalfe KA, Esplen MJ, Goel V, et al. Predictors of quality of life in women with a bilateral prophylactic mastectomy. Breast J 2005;11:65-9. [Crossref] [PubMed]
- Rowland E, Metcalfe A. A systematic review of men's experiences of their partner's mastectomy: coping with altered bodies. Psychooncology 2014;23:963-74. [Crossref] [PubMed]


