Capsular contracture in implant based breast reconstruction—the effect of porcine acellular dermal matrix
Introduction
Acellular dermal matrices (ADMs) are increasingly used in immediate implant-based breast reconstruction (BR) (1). Although originally intended to provide support and coverage of the breast lower pole, growing evidence suggests that ADM use may also mitigate the risk of capsular contracture (CC) (2-5).
CC is a significant complication in implant-based reconstructions, with a cumulative incidence of 6–18%, over a 3–6-year period reported in core clinical studies of implant manufacturers (6-8). In contrast, ADM-assisted reconstructions have consistently been associated with CC rates of <5%, albeit a shorter follow-up period of 0.6–2.4-year (9-15) is reported. Mechanistic studies in animal models as well as human histopathologic studies suggest a reduction or delay in capsule formation in the presence of ADM as a possible explanation for the reduced occurrence of CC (5,16-18).
External beam radiotherapy (EBRT) is a recognised risk factor for CC and CC rates of 15–50% have been reported in standard submuscular reconstructions without the use of ADM (19-24). Given the rising trend of immediate implant-based BR following mastectomy before the need for radiotherapy is fully determined, there is an increasing need for novel measures to reduce CC risk in this patient group. Because ADMs appear to mitigate the risk of CC, the natural question is whether these matrices also reduce the risk in the setting of radiotherapy. Data on the incidence of CC in the setting of radiotherapy in ADM-assisted reconstructions are, however, limited with short follow up times (9,12,13,18,24-28). Salzberg recently published a long-term follow-up (13 years) with low CC rate even after radiotherapy (1.9%) when ADM is used in one-stage implant based BR (28). The purpose of this study was to compare CC rates in non-irradiated and irradiated porcine acellular dermal matrix (PADM)-assisted one- and two-stage immediate implant-based BR.
Methods
This is a retrospective cohort study performed from December 2008 to October 2012 at Guy’s and St Thomas’s Hospitals, London. During this period, 149 patients (200 reconstructions) who underwent immediate, implant-based BR with the assistance of PADM with or without radiotherapy were identified.
Our operative technique of using PADM (StratticeTM, LifeCell Corporation, Branchburg, NJ, USA) has been previously described (29). Briefly, mastectomy was performed via a skin-sparing or nipple-sparing approach by breast surgeons in attendance of the plastic surgeon. Following mastectomy, the pectoralis major muscle was elevated and a subpectoral pocket was created using standard techniques. PADM was rinsed in antibiotic solution prior to insertion. The inferior border of PADM was sutured to the chest wall along the inframammary fold, extending medially and laterally. A silicone cohesive gel implant (one stage) or tissue expander (two stage) was placed into the pocket and the upper border of PADM was sutured to the inferior border of the freed pectoralis muscle in a sublay technique resulting in closure of the pocket. Extra care was taken to avoid creases or folds of the PADM and dead space between the PADM and host tissue. Two drains were placed, one in the pocket and the other subcutaneously along the inframammary fold. If axillary clearance was performed, a third drain was placed in the axilla. All drains were removed after drainage was reduced to <30 mL over 24 h. Typically, prophylactic intravenous antibiotics were commenced 30 min prior to surgery, which was followed by 3 additional intravenous doses and then switched to oral antibiotics, which were continued for 5 days.
In one stage procedures, a definitive implant was placed at the same operative step as mastectomy was performed. In the two stage procedures, an expander was placed at first step (critical skin flap perfusion, surgeons’ preference, patients wish for larger breast size), followed by weeks of expansion to the desired breast size.
Adjuvant anthracycline or anthracycline/taxane based chemotherapy given over 6–8 cycles generally started 4 weeks postoperatively (one stage) or after completion of expansion (two stage) when no wound healing problems were present. In a neoadjuvant chemotherapy setting a time slot of 3–6 weeks was maintained before mastectomy and reconstruction. Adjuvant radiotherapy was initiated at a minimum of 3 weeks following completion of chemotherapy or surgery. External beam irradiation to the chest wall comprised 40 Gy in 15 fractions delivered using tangential 6 and/or 10MV photon beams. In two stage procedures EBRT and chemotherapy were started after completion of expansion and the exchange for the definitive implant was performed after completion of chemo/radiotherapy in all cases.
We included patients with a minimum postoperative follow up of 6 months after insertion of implant in one-stage procedures and after exchange of expander for definitive implant in two-stage procedures in non-irradiated BR. Irradiated BR were included with a minimum follow up time of one year from completion of radiotherapy.
Data collection and analysis
Patient demographic data including age, body mass index, diabetes, current tobacco use, chemotherapy (neoadjuvant or adjuvant) and radiotherapy use were collected from hospital records. Clinical examination and classification of CC was performed in the outpatient clinic by the first author only (AML who was not involved in the surgery). CC was graded using the conventional Spear-Baker classification for non-irradiated breasts (30). A modified version of this classification in irradiated breasts (26). Grade III and IV CC were determined as clinically significant CC according to the literature.
All complications occurring during the follow-up period specified above were recorded. Complications and Grade III/IV CC rates were analysed using a Generalized Estimating Equations (GEE) model (31). This model accounts for potential intrapatient correlation of results. If due to low cell sizes a GEE model could not be fitted rates were compared using Fisher’s exact test. Exact confidence intervals for rates and rate differences were calculated assuming a binomial distribution. A value of P<0.05 was considered significant.
Results
Over a 4-year period, 149 patients underwent 200 PADM-assisted immediate implant-based BR. A total of 177 reconstructions met our inclusion criteria (>6-month follow-up after the last procedure in non-irradiated breasts and >1-year follow-up after radiotherapy in irradiated breasts). Of the 177 reconstructions, 55 were excluded because 28 were switched to autologous reconstruction or had implant loss (infections, skin flap necrosis, patients wish) and 27 were lost to follow up. The remaining 122 reconstructions were included in this study. Eighty mastectomies were performed for oncological reason, 42 were risk-reducing mastectomies in BRCA positive patients. Sixty-five were one-stage (direct to implant) and 57 two-stage procedures. In 84 BR, no radiotherapy had to be administered, 38 BR underwent postoperative radiotherapy. Patient demographics are summarized in Table 1.
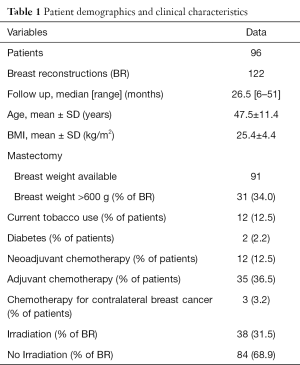
Full table
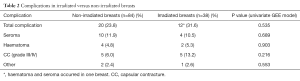
Patients were followed for a median of 26.5 months (range, 6–51 months). During this time, complications occurred in 32 breasts for a total complication rate of 26%. Complications included, 14 seromas (11.5%), 6 haematomas (7.3%), and 10 grade III/IV CC (8.2%) and 3 others (2.4%) (1 small skin necrosis, 1 implant rotation, 1 moderate wound healing problem).
When stratified by radiotherapy use, total complication rate and CC rate was numerically higher 12 (31.6%) vs. 20 (23.8%), but not significantly higher (P=0.535), in irradiated breasts (Table 2). Patient demographics (age) and clinical characteristics (BMI, tobacco use, diabetes, breast weight) were similar between the irradiated and non-irradiated groups (Table 3). Mean follow up was significantly longer (31.8 months) in irradiated versus non-irradiated breasts (22.2 months) due to the inclusion criteria (follow up time in irradiated BR at least >1 year of termination of radiotherapy, compared to minimum 6 months postoperative in non-irradiated BR). A significantly higher percentage of breasts in the irradiated group had mastectomy weights >600 g. Treatment with neoadjuvant and adjuvant chemotherapy was also significantly higher in irradiated breasts (Table 3).
Full table

Full table
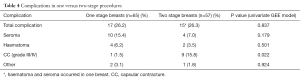
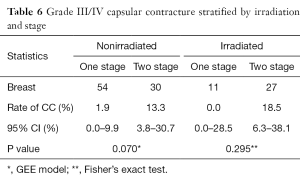
When stratified by one- vs. two-stage procedures, overall complications were similar between the groups while seroma and hematoma were numerically higher and CC significantly lower in one-stage procedures (1.5% vs. 15.8%, P=0.022) (Table 4). There was no difference in patient demographics and clinical characteristics between the two groups, except for adjuvant chemotherapy and adjuvant radiotherapy use, which was significantly higher in two-stage procedures (Table 5). Average follow-up was significantly longer in two-stage procedures (31.0 vs. 21.6 months, P<0.001) because of increasing numbers of one-stage procedures toward the end of the study period. The higher rate of CC in two-stage procedures was seen in both irradiated and non-irradiated breasts (Table 6).
Full table

Full table
Full table
The univariate analysis for exploration of risk factors of the occurrence of clinical CC (Grade III/IV) showed two significant risk factors in this study: follow up time (P<0.001) and stages (P=0.022) (Table 7). Irradiation and chemotherapy did not point out as significant risk factors, neither BMI, age, breast size or smoking.
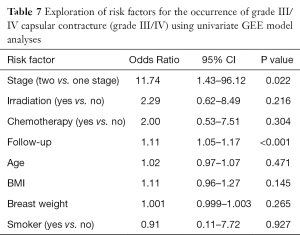
Full table
On the multivariate analysis combining follow up time and the stages (Table 8), the follow up time kept a significant (P=0.013) influence on occurrence of CC in this study. The two-stage procedure remained a strong risk factor for development of CC (P=0.093)
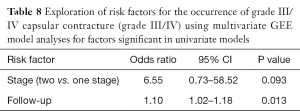
Full table
Discussion
CC is a significant complication of implant-based, BR with unpredictable occurrence and recurrence despite treatment. The aetiology of CC remains unclear but recent evidence suggests a bacterial infection trigger for the inflammatory response that characterizes CC (32). There are no known specific preventive measures for CC but technical considerations such as antibiotic breast pocket irrigation (33), use of textured implants (34), and subpectoral placement of implants (35) have all been shown to reduce the risk of contracture. A growing body of evidence indicates that the use of ADM may provide another means to reduce the risk of CC (2-5,9-15).
Radiotherapy is considered as one of the most important factors that augment the risk of CC. In the absence of radiotherapy, the rate of clinically significant CC (Grade III/IV) varies from 0–20% in standard submuscular BR without the use of ADM. In the presence of radiotherapy, the rate increases to 15–50%, which is an approximately 4-fold increase (19-24). The need for subsequent irradiation is, thus, often considered as a relative contraindication for implant-based BR. Radiation-induced CC is undoubtedly detrimental to the aesthetic appearance of the implant-enhanced breast.
Data on the rate of CC in ADM-assisted reconstructions in the setting of radiotherapy are limited. In the published studies the contracture rate varied between 0–60% (9,12,13,18,24-28) and 0–3% for non-irradiated breasts (12,25,26).
In the present study using PADM for lower pole reinforcement, we found a low rate of clinically significant CC (grade III/IV) in both non-irradiated (6%) and irradiated (13%) immediate implant-based BR, although the rate was 2-fold higher in the irradiated group. The median follow up was almost 2 years in the non-irradiated group and almost 3 years in the irradiated group, suggesting that the low risk of CC with ADM use is at least maintained over medium term.
The findings of this study have been attenuated by the fact that the longer follow up time after primary surgery (one stage) or after exchange of the expander for definitive implant (two stage) the higher risk for occurrence of high grade CC was found. This fact suggests a further increase in CC in long term. On the other hand, Salzberg in his recent study found CC, when ADM was used, did appear during the first 2 years postoperatively and was described as an early event in ADM based reconstructions (28). Nevertheless, there is a need for more large series with long follow up times and comparison to non-ADM reconstructions to strengthen the findings.
Regardless a significant increase in occurrence of CC was found in expander based traditional (two stage) procedures compared to direct to implant (one stage) reconstructions (P=0.022). In the multivariate analysis, this trend was maintained after clearing for the effect of follow up time (P=0.093) and hasn’t been described in literature to our knowledge. Two-stage procedures provide the opportunity to perform capsulectomy at the time of exchange of the expander (which eventually also was exposed to chemo/radiotherapy) for the definitive implant. Therefore, it is generally believed that two stage procedures are associated with a lower incidence of CC. Our results seem to contradict these general expectations. It is imaginable that tissue expansion in itself increases scar tissue/capsule formation similar to callus formation during healing of fractures. The repetitive trauma of expansion, tension and weakening of the skin flap may have created an environment for an exaggerated tissue response to the implant, which may explain the higher rate of contracture in our series.
This study is limited by its retrospective nature, the small number of reconstructions in the irradiated group, and the absence of a non-ADM group and the relatively short follow up times. Nevertheless, compared to published CC rates with similar follow up times, our rates are substantially lower, suggesting that PADM use is associated with a reduced or delayed risk for CC and could tribute to longevity of the reconstruction result. In addition, this study points out the importance of follow up times concerning occurrence of CC and suspects expansion to trigger CC.
Conclusions
Our data support the current clinical evidence that ADM use in implant-based BR is associated with a reduced risk of CC when compared to the standard submuscular techniques in literature. The reduced risk is maintained in the setting of radiotherapy. Two stage procedures in our study population showed increased grade III/IV CC compared to one stage procedures with or without exposure to radiation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Statement of institutional review board approval and/or statement of conforming to the Declaration of Helsinki. This study was approved by the local Institutional Review Board.
References
- Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg 2013;70:103-10. [Crossref] [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [Crossref] [PubMed]
- Spear SL, Seruya M, Clemens MW, et al. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg 2011;127:1047-58. [Crossref] [PubMed]
- Clough KB, O'Donoghue JM, Fitoussi AD, et al. Prospective evaluation of late cosmetic results following breast reconstruction: I. Implant reconstruction. Plast Reconstr Surg 2001;107:1702-9. [Crossref] [PubMed]
- Stump A, Holton LH 3rd, Connor J, et al. The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plast Reconstr Surg 2009;124:82-91. [Crossref] [PubMed]
- Stevens WG, Harrington J, Alizadeh K, et al. Five-year follow-up data from the U.S. clinical trial for Sientra's U.S. Food and Drug Administration-approved Silimed® brand round and shaped implants with high-strength silicone gel. Plast Reconstr Surg 2012;130:973-81. [Crossref] [PubMed]
- Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [Crossref] [PubMed]
- Spear SL, Murphy DK, Slicton A, et al. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg 2007;120:8S-16S; discussion 17S-18S.
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [Crossref] [PubMed]
- Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg 2009;123:1-6; discussion 107-8. [Crossref] [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-410e. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Israeli R, Feingold RS. Acellular dermal matrix in breast reconstruction in the setting of radiotherapy. Aesthet Surg J 2011;31:51S-64S. [Crossref] [PubMed]
- Cassileth L, Kohanzadeh S, Amersi F. One-stage immediate breast reconstruction with implants: a new option for immediate reconstruction. Ann Plast Surg 2012;69:134-8. [Crossref] [PubMed]
- Hanna KR, DeGeorge BR Jr, Mericli AF, et al. Comparison study of two types of expander-based breast reconstruction: acellular dermal matrix-assisted versus total submuscular placement. Ann Plast Surg 2013;70:10-5. [Crossref] [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010;126:1842-7. [Crossref] [PubMed]
- Komorowska-Timek E, Oberg KC, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg 2009;123:807-16. [Crossref] [PubMed]
- Moyer HR, Pinell-White X, Losken A. The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg 2014;133:214-21. [Crossref] [PubMed]
- Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg 2000;105:930-42. [Crossref] [PubMed]
- Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 2006;118:832-9. [Crossref] [PubMed]
- Percec I, Bucky LP. Successful prosthetic breast reconstruction after radiation therapy. Ann Plast Surg 2008;60:527-31. [Crossref] [PubMed]
- Persichetti P, Cagli B, Simone P, et al. Implant breast reconstruction after salvage mastectomy in previously irradiated patients. Ann Plast Surg 2009;62:350-4. [Crossref] [PubMed]
- Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg 2011;128:353-9. [Crossref] [PubMed]
- Ho AL, Bovill ES, Macadam SA, et al. Postmastectomy radiation therapy after immediate two-stage tissue expander/implant breast reconstruction: a University of British Columbia perspective. Plast Reconstr Surg 2014;134:1e-10e. [Crossref] [PubMed]
- Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg 2012;129:1223-33. [Crossref] [PubMed]
- Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 2012;130:1-9. [Crossref] [PubMed]
- Weichman KE, Cemal Y, Albornoz CR, et al. Unilateral preoperative chest wall irradiation in bilateral tissue expander breast reconstruction with acellular dermal matrix: a prospective outcomes analysis. Plast Reconstr Surg 2013;131:921-7. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Lardi AM, Ho-Asjoe M, Mohanna PN, et al. Immediate breast reconstruction with acellular dermal matrix: factors affecting outcome. J Plast Reconstr Aesthet Surg 2014;67:1098-105. [Crossref] [PubMed]
- Spear SL, Baker JL Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg 1995;96:1119-23; discussion 1124. [Crossref] [PubMed]
- Aitkin MA. Statistical modelling in GLIM. Oxford [Oxfordshire]: Oxford University Press; New York: Clarendon Press, 1989.
- Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg 2010;126:835-42. [Crossref] [PubMed]
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg 2006;118:46S-52S. [Crossref] [PubMed]
- Burkhardt BR, Eades E. The effect of Biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg 1995;96:1317-25. [Crossref] [PubMed]
- Embrey M, Adams EE, Cunningham B, et al. A review of the literature on the etiology of capsular contracture and a pilot study to determine the outcome of capsular contracture interventions. Aesthetic Plast Surg 1999;23:197-206. [Crossref] [PubMed]


