Patients’ satisfaction with anatomic polyurethane implants
Introduction
Silicone gel mammary implants have been utilized in plastic surgery procedures since 1962 when Cronin and Gerow used the first mammary implant and subsequently presented their results in 1964 at the Third International Congress of Plastic Surgery in Amsterdam (1). Six years later, Ashley presented the silicone implant with a polyurethane cover, which was intended to prevent the frequent occurrence of capsular contractures (2,3). Since then, many authors have used and studied this type of breast implant (4-28). Forty-five years later, these implants are still marketed in most countries in America, Europe, Asia, Africa, and Oceania with the development and evolution of the gels and elastomer.
Implant designers began to experiment with a rough silicon cover, similar to that composed of polyurethane materials and introduced the textured implant in the 1980s. By the 1990s, anatomic implants with both polyurethane and textured covers were being produced.
Materials and methods
From 2004 to 2014, we performed surgical interventions on 525 patients using anatomic implants with polyurethane covers. Among these, 370 were primary surgeries, and 155 were revisions for capsular contractures, rupture, volume changes and incorrect positioning of the implant.
Volumes range was between 255 and 375 cc were used with a mean of 325 cc.
The mean age of the patients was 52±11 years [range, 30–76 years; standard deviation (SD)] .
Mean follow-up time, defined as the mean interval between the operation and the most recent follow-up evaluation, was 70 months (range, 20–120 months).
All the implants used were anatomical, round base, high and extra high projection, Silimed (Brazil).
Surgical technique
Implants were chosen on the basis of mammary width and height measurements. The chosen devices were 1 cm shorter than the mammary measurements in each of these dimensions. Implant projection was selected according to skin stretch and patient desire, varying only between high and extra high projections.
General anesthesia was used in the 100% of the cases.
Intravenous cephalosporin-based antibiotic was administered 30 minutes before the surgery, as single doses.
Three hundred and seventy four cases, 289 primary breast augmentation and 85 secondary cases were made by an inferior hemi areolar incision at 2 mm from the inferior edge to allow posterior myorraphy of the areolar smooth muscle (28); the other 151 cases, 90 primary breast augmentation and 61 secondary cases were approached by an infra mammary fold incision.
In the primary augmentation patients, the pocket plane was always partially subfascial as explained in our previously published report in 2007 (29). In the revisions cases same retropectoral pocket was retained in 45 cases and in another 40 cases, the pocket was changed from prepectoral to retropectoral.
Drains were used in all ours secondary cases to reduce the dead space by the negative pressure of the suction, after the capsulectomy. In primary augmentation, we try not to use because tissue adherence to implant surface is one of the polyurethane cover characteristics (26,29).
PDS™ (3/0 and 5/0) was used to close the incision and a compressive bandage during the first 48 hours, continued with a brassiere during the next 2 weeks. Driving was forbidden for 10 days. After 21 days, some type of exercise was permitted, such as walking without involvement of the pectoral muscles. There was no physical restriction after 45 days.
Silimed™ has three different models of anatomic polyurethane breast implants with respect to the base. The nuance model has a horizontal axis that is greater than the vertical axis. The enhance model has a vertical axis is greater than the horizontal axis. The base is round in the natural model.
We prefer the Silimed™ Biodesign natural model, which has a round base, as well as both the high and extra high profiles, depending on the previously mentioned factors.
Currently there are two companies that manufacture implants with polyurethane cover, Silimed™ and Polytech™. In this series, we only used Silimed™ in order to have an accurate assessment.
Statistical analysis
Continuous variables are described as mean with SD or medians with range. Statistical analysis was conducted with OpenEpi (United States Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA, USA) to calculate 95% confidence intervals (CIs).
Results
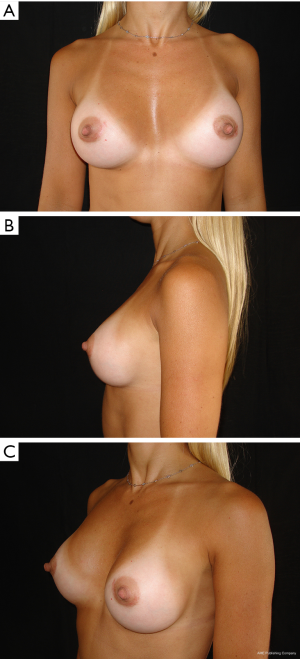
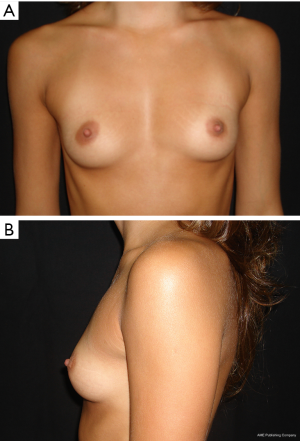
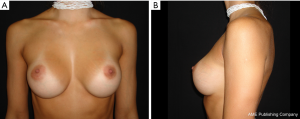
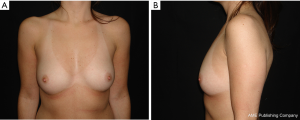
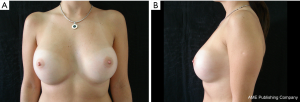
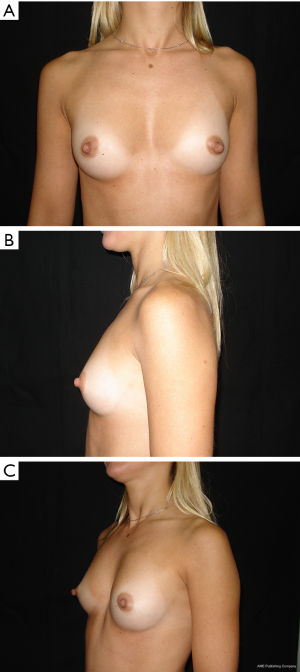
The results are aesthetically natural with great satisfaction of patients (Figures 1-6).
In 17 (3.23%) patients, one or more post-operative complications occurred.
The percentages are: hematoma (1.5%), infection (0.4%), and skin rash (1.4%). Sometimes skin rashes occurred (29). It is important to note that there is an increase in temperature and itchiness of the affected area that differentiates from an infectious process.
Patient satisfaction
Patient satisfaction was assessed based on their perceptions of several aesthetic aspects while dressed, undressed, general satisfaction and body image perception. Patients were asked to rate their responses to the four questions regarding their surgery and satisfaction with their appearance by email 12 months after surgery.
We used a Likert (30) scale: 5, excellent; 4, very good; 3, good; 2, fair; and 1, bad (Table 1).

Full table
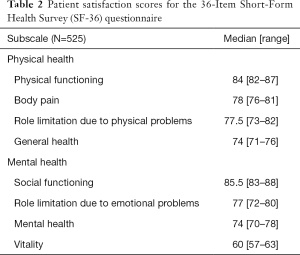
The 36-Item Short-Form Health Survey (SF-36) questionnaire, the well-known, validated questionnaire for quality-of-life assessment, was administered 3 months after surgery. This questionnaire evaluates health status with two separate components: mental health and physical health. The physical health component includes four scales comprising ten questions about physical functioning (PF), four questions about role limitation due to physical problems (RP), two questions about body pain (BP) and five questions about general health (GH). The mental health component also includes four scales. These comprise four questions about vitality (VT), two questions about social functioning (SF), three questions about role limitation due to emotional problems (RE), five questions about mental health (MH) and one question about general health perception. Each of these eight subscales is scored separately, from 0 to 100. A higher score in each subscale indicates a better condition (Table 2).
Full table
General patient satisfaction while dressed was excellent in 75% and very good in 20% of cases, while the patient satisfaction when undressed was excellent in 98% of the cases. Regarding the specific breast features, patients frequently reported excellent or very good results with regard to shape (98%), size (91%), texture (97%), and symmetry (98%).
In secondary cases (Table 3), interestingly, satisfaction regarding the shape was 99% when compared to the patients’ previous round implants. They stated that the shape did not appear as artificial as round implants and their breasts almost looked natural when dressed and naked.
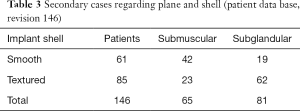
Full table
Discussion
When planning augmentative mammary surgery, there are certain details about the patient’s biotype that should be observed in order to obtain good aesthetic results. These include the thorax (i.e., concavity, convexity, and asymmetry as well as the presence of hemi-thorax anomalies), submammary crease (i.e., shape and distance to the areola), nipple-areola complex (NAC; size and shape), elasticity of the skin, breast tissue coverage (i.e., pinch test) and any type of asymmetry.
These details should be observed in any type of augmentative surgery regardless of the surface or form receiving the implant. With the use of an anatomic implant, important details on the patient’s desired type of projection, the implant’s cohesion, and the correct alignment of both vertical and horizontal axes should be observed in order to achieve good results.
The type of breast projection that is desired by the patient should be considered. Projection of the breast refers to the distance separating the surface of the pectoralis major muscle and the base of the nipple. A variety of profiles are available in order to achieve the patient’s desired shape.
The cohesive forces within the gel are also important factors that determine the natural appearance and persistence of form through time. It is very important to know the difference between cohesion and firmness, with the former referring to adherence and the latter, to compaction of particles.
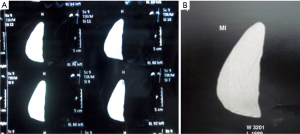
High cohesive gel anatomical implants maintain shape in vivo, with the possibility of checking it by MRI studies (Figure 7).
Textured anatomic implants may rotate along their vertical axis as time passes. This has been documented in different publications where rotation occurred in 20% of the cases reported by Baeke (31) and in 0.4% to 1% of the cases reported by Hammond et al. (32-34). Although the rates varied in their individual studies, they agreed that anatomic texturized implants might result in unexpected rotations, which can produce an aesthetic deformation of the breast. Therefore, polyurethane cover anatomical implants assure the patient and the surgeon to prevent this complication.
Nowadays, patient paradigm for breast augmentation has changed, asking today not only for volume but also natural shape.
Anatomical implants provides the possibility of modifying shape in the most difficult cases like pseudo ptosis, congenital pathologies such as Poland syndrome, or during mammary reconstruction. All the mentioned are precise indications for anatomic implants with polyurethane cover.
The polyurethane creates a histologically vascularized capsule due to the inflammatory reaction, and is not as fibrous as the smooth or textured implants. Based on a capsular architecture that is totally different from textured or smooth implants, obtaining a soft breast allows the surgeon to place the implant correctly with the help of the Velcro™ effect.
During our 27 years of performing these procedures using polyurethane implants, we have published our observations on the histology and immunology of the capsule, modifications of the anatomic implant over time, and methodology in leading scientific journals. These publications provide additional information for surgeons who are using polyurethane breast implants.
In breast reconstruction, given that most currently used tissue expanders are anatomically shaped and the surgical time that is needed in order to replace the expander with an anatomical implant, the subsequent capsular contracture range is estimated to occur between 10% and 40% of cases. For this reason, polyurethane anatomical implant covers are highly recommended. It is also useful in skin sparing mastectomy and immediate one-step breast reconstruction with implants (35).
This process imparts a textured appearance and the capsule has a very different histological architecture, which maintains the implant in position even after reabsorption of the polyurethane foam. Many surgeons are aware of the dangers from the polyurethane foam degradation products, as identified in Chan’s paper in 1991 (36). In 1994, the FDA commissioned study described by Hester et al. illustrated the significant failures of Chan’s study. This led to the release of the 1995 FDA statement (37) after Santerre communication in the 24th Annual Meeting of the Society for Biomaterials (38). Since then, the safety of polyurethane in breast implants is no longer discussed, and they are still in use after 45 years. The published works are only hypotheses that are not based on any scientific evidence and refer to the old implants. The new generation of implants [1992–1993] have positive changes, including a cohesive gel, vulcanized polyurethane foam, new shape and optimal manufacturing systems (39).
There is no foundation, other than speculation to be cautious over oncologic risk for polyurethane cover implants. Several papers covered this topic (40).
Anatomical polyurethane cover implants have a reduced risk of the most frequent problems: late seroma, capsular contracture and anatomical implant rotation.
Conclusions
Women requesting breast implants augmentation are aware of anatomical devices risks as: rotation, capsular contracture and revisions.
Information about late seroma and anaplastic large cell lymphoma (ALCL) risks regarding this surgery is easily available for every potential patient.
Anatomical polyurethane breast implants have not been associated in literature with late seroma and ALCL.
High rate of patient satisfaction has been obtained in our series with the use of anatomically covered polyurethane implants.
We strongly believe that covered polyurethane implants are a great option for augmentative mammoplasty, whether primary or revisionary surgery.
Patient education and information is a heavy task, and the informed consent documentation must be supported with real data. Polyurethane anatomical cover implants provides the mentioned benefit for both, patient and doctor.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cronin T, Gerow F. Augmentation mammoplasty: a new natural feel prosthesis. Transactions of the Third International Congress of Plastic Surgery. Amsterdam: Excerpta Medica Foundation, 1964:41-9.
- Ashley FL. A new type of breast prosthesis. Preliminary report. Plast Reconstr Surg 1970;45:421-4. [Crossref] [PubMed]
- Ashley FL. Further studies on the natural-Y breast prosthesis. Plast Reconstr Surg 1972;49:414-9. [Crossref] [PubMed]
- Barone FE, Perry L, Keller T, et al. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg 1992;90:77-86. [Crossref] [PubMed]
- Brand G. Foam-covered mammary implants. Clin Plast Surg 1984;73:498. [PubMed]
- Capozzi A. Polyurethane-covered gel mammary implants. Plast Reconstr Surg 1982;69:904. [Crossref] [PubMed]
- Capozzi A, Pennisi VR. Clinical experience with polyurethane-covered gel-filled mammary prostheses. Plast Reconstr Surg 1981;68:512-20. [Crossref] [PubMed]
- Cocke WM, Leathers HK, Lynch JB. Foreign body reactions to polyurethane covers of some breast prostheses. Plast Reconstr Surg 1975;56:527-30. [Crossref] [PubMed]
- Cohney BC, Cohney TB, Hearne VA. Nineteen years' experience with polyurethane foam-covered mammary prosthesis: a preliminary report. Ann Plast Surg 1991;27:27-30. [Crossref] [PubMed]
- Dolsky RL. Polyurethane-coated implants. Plast Reconstr Surg 1985;76:974-5. [Crossref] [PubMed]
- Eyssen JE, von Werssowetz AJ, Middleton GD. Reconstruction of the breast using polyurethane-coated prostheses. Plast Reconstr Surg 1984;73:415-21. [Crossref] [PubMed]
- Gasperoni C, Salgarello M, Gargani G. Polyurethane-covered mammary implants: a 12-year experience. Ann Plast Surg 1992;29:303-8. [Crossref] [PubMed]
- Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 2006;117:757-67; discussion 768-72. [Crossref] [PubMed]
- Handel N, Silverstein MJ, Jensen JA, et al. Comparative experience with smooth and polyurethane breast implants using the Kaplan-Meier method of survival analysis. Plast Reconstr Surg 1991;88:475-81. [Crossref] [PubMed]
- Herman S. The Même implant. Plast Reconstr Surg 1984;73:411-4. [Crossref] [PubMed]
- Hester TR Jr, Nahai F, Bostwick J, et al. A 5-year experience with polyurethane-covered mammary prostheses for treatment of capsular contracture, primary augmentation mammoplasty, and breast reconstruction. Clin Plast Surg 1988;15:569-85. [PubMed]
- Hester R. Discussion, diagnosis and treatment of complications occurring with polyurethane-covered breast implants. Plast Reconstr Surg 1990;86:711.
- Hoefflin SM. Extensive experience with polyurethane breast implants. Plast Reconstr Surg 1990;86:166-7. [Crossref] [PubMed]
- Melmed EP. Polyurethane implants: a 6-year review of 416 patients. Plast Reconstr Surg 1988;82:285-90. [Crossref] [PubMed]
- Melmed EP. Treatment of breast contractures with open capsulotomy and replacement of gel prostheses with polyurethane-covered implants. Plast Reconstr Surg 1990;86:270-4. [Crossref] [PubMed]
- Pennisi VR. Long-term use of polyurethane breast prostheses: a 14-year experience. Plast Reconstr Surg 1990;86:368-71. [Crossref] [PubMed]
- Pennisi VR. Polyurethane-covered silicone gel mammary prosthesis for successful breast reconstruction. Aesthetic Plast Surg 1985;9:73-7. [Crossref] [PubMed]
- Pitanguy I, Brentano J, Castro Ramalho M, et al. Implante du silicone gel com revestimento du poliuretano. Rev Brasil Cirurgia 1990;80:119.
- Schatten WE. Reconstruction of breasts following mastectomy with polyurethane-covered, gel-filled prostheses. Ann Plast Surg 1984;12:147-56. [Crossref] [PubMed]
- Sinclair TM, Kerrigan CL, Buntic R. Biodegradation of the polyurethane foam covering of breast implants. Plast Reconstr Surg 1993;92:1003-13; discussion 1014. [Crossref] [PubMed]
- Vázquez G. A ten-year experience using polyurethane-covered breast implants. Aesthetic Plast Surg 1999;23:189-96. [Crossref] [PubMed]
- Vázquez G. Modification of the anatomic silicone gel implant with polyurethane cover. Cir Plast Iberolatinamer 2005;31:193-8.
- Vázquez G, Moretti E, Pellón A, et al. The Importance of the Areolar Smooth Muscle in Augmentation Mastoplasty. Aesthetic Plast Surg 2009;33:298-301. [Crossref] [PubMed]
- Vázquez G, Pellón A. Polyurethane-coated silicone gel breast implants used for 18 years. Aesthetic Plast Surg 2007;31:330-6. [Crossref] [PubMed]
- Likert R. A technique for the measurement of attitudes. Archives of Psychology 1932;140:1-55.
- Baeke JL. Breast deformity caused by anatomical or teardrop implant rotation. Plast Reconstr Surg 2002;109:2555-64; discussion 2568-9. [Crossref] [PubMed]
- Tebbetts JB. Patient acceptance of adequately filled breast implants using the tilt test. Plast Reconstr Surg 2000;106:139-47; discussion 148-9. [Crossref] [PubMed]
- Spear S, Willey S, Robb GL, et al. editors. Surgery of the breast: principles and art. Philadelphia; Lippincott Williams & Wilkins, 2006.
- Hedén P, Jernbeck J, Hober M. Breast augmentation with anatomical cohesive gel implants: the world's largest current experience. Clin Plast Surg 2001;28:531-52. [PubMed]
- Rancati A, Soderini A, Dorr J, et al. One-step breast reconstruction with polyurethane-covered implants after skin-sparing mastectomy. J Plast Reconstr Aesthet Surg 2013;66:1671-5. [Crossref] [PubMed]
- Chan SC, Birdsell DC, Gradeen CY. Detection of toluenediamines in the urine of a patient with polyurethane-covered breast implants. Clin Chem 1991;37:756-8. [PubMed]
- Food and Drug Administration (FDA). Update: Study of TDA released from polyurethane foam-covered breast implants. 1995.
- Santerre JP, Wang FG, Labor RS. Biodegradation of the microthane polyester polyurethane by the lissome enzyme cholesterol esterase and identification of degradation products. 24th Annual Meeting of the Society for Biomaterials. San Diego, CA: 1998.
- Pan SY, Lavigne E, Holowaty EJ, et al. Canadian breast implant cohort: extended follow-up of cancer incidence. Int J Cancer 2012;131:E1148-57. [Crossref] [PubMed]
- Frame JD. Commentary on: Breast implants and the risk of breast cancer: a meta-analysis on cohort studies. Aesthet Surg J 2015;35:63-5. [Crossref] [PubMed]