Impact of value based breast cancer care pathway implementation on pre-operative breast magnetic resonance imaging utilization
Introduction
In the past decade, the use of bilateral breast magnetic resonance imaging (MRI) has been increasingly adopted as a supplemental tool for the pre-surgical evaluation of breast cancer (BC). Previous studies demonstrate that women diagnosed with early BC were found to have histology showing additional foci in the affected breast in 20–60% of cases (1-4), but the clinical benefit of identifying these extra lesions is very limited. MRI is known to have increased sensitivity as compared to conventional mammography and ultrasound, ranging from 79% to 100% (5) and is not lessened by breast density (5). MRI has been broadly utilized pre-operatively to determine the extent of disease in the affected breast and also to detect occult disease in the contralateral breast (6-8). Pre-operative MRI has been thought by some to improve surgical planning (1,6,9), decrease re-excision rates (1,10), and reduce recurrences (11). Nonetheless, it has been highly debated if MRI should be routinely used in the diagnostic evaluation of all BC (12,13).
Studies have suggested benefit in the selective use of MRI. These situations include evaluation of patients with invasive lobular carcinoma (ILC), which can be difficult because of a unique growth pattern and an increased risk of contralateral BC (14,15). Women with dense breasts have decreased mammography sensitivity which can also pose a challenge (5,16). MRI should also be used in planning for partial breast irradiation because these patients will not receive the sterilizing effect of whole breast irradiation (17). Patients with isolated axillary presentation and unknown primary origin pose a unique challenge and may benefit from an MRI (18). Patients with genetic mutations associated with increased risk of BC also have an increased risk of contralateral BC and should undergo breast MRI for treatment planning (19). Breast MRI has also been shown to have better accuracy in detecting tumor response in patients undergoing NAC (20).
In light of rising health care costs, value-based care has become a priority for health care organizations (21,22). The goal of value based care is to improve patient care outcomes while reducing costs (21). In April 2014, Cleveland Clinic developed an institution wide care path initiative. BC care paths were developed by multidisciplinary expert consensus at Cleveland Clinic, evaluating available evidence based data and national guidelines. Care paths were developed for screening, high risk, and diagnosis and treatment of BC. Care paths are meant to guide care, but are not intended to replace individual care team decision making for all patients. Providers make patient decisions about care, and utilization is monitored. The ordering of pre-operative breast MRI is not regulated in any way and there are no incentives built into the implementation.
The BC care path for use of MRI recommends against the routine use of pre-operative MRI in every newly diagnosed BC patient. MRI use is recommended in the care path for patients with invasive lobular cancer (ILC) (14,15), candidates for neo-adjuvant chemotherapy (20), heterogeneously or extremely dense mammographic tissue (5,16), malignancy of unknown primary origin (18), candidates for partial breast irradiation (17), or suspicion of contralateral BC (i.e., pathogenic genetic mutation) (19).
Care paths were routinely utilized in our multidisciplinary tumor board and were regularly discussed and reviewed at staff meetings. Additionally, care path indications for pre-operative MRI were included as a drop-down into a standard surgical note in the electronic medical record introduced during this time period in conjunction with care path recommendations.
We compared the number of pre-operative MRIs ordered before and after implementing an institutional wide BC care path.
Methods
We performed a retrospective Institutional Review Board approved study looking at all patients diagnosed with invasive BC or ductal carcinoma in situ (DCIS) at Cleveland Clinic during the years 2012, 2014, and January through May 2015. The care path was implemented and published online in April 2014. The year 2013 was not evaluated as a baseline as care paths were being developed and discussed. Practice patterns were beginning to change before implementation. It was felt that 2012 would be a more appropriate baseline view of practice patterns. Patients were excluded if the initial consultation was not done by one of the surgeons practicing primarily at our institution’s main campus where BC care paths were initially implemented. For the purposes of this analysis, only the patients treated at the main campus were analyzed as the impact of care path implementation was felt to be greatest with the surgeons practicing at this location. Secondly, patients were excluded if a breast MRI was already ordered by an outside physician prior to surgical consultation.
The decision for pre-operative MRI was made by the treating team and was not randomized. Information collected included patient demographics, presence of genetic mutations, tumor characteristics, mammographic density, and whether neo-adjuvant chemotherapy was given. Genetic mutations included pathogenic variants in both highly penetrant genes and moderate risk genes. Dense breasts were defined as heterogeneously dense or extremely dense on mammogram as reported by dedicated breast radiologists. Patients referred from outside institutions did not always have mammographic breast density information available. These patients were included in the analysis. Breast MRI was performed at either of two centers within our institution. All breast imaging, including mammograms, ultrasounds, and MRI were interpreted by a dedicated group of breast radiologists over all time periods. During the time period in question, there was no change in the availability of MRI.
Continuous variables were described by means, standard deviations, and categorical variables by counts and percentages. A model of the likelihood of having a pre-operative MRI by time was fit using a logistic generalized linear mixed model and accounts for surgeon-to-surgeon variability. This allowed the intercept and slope to vary from surgeon-to-surgeon. Predictions for the probability of having a pre-operative MRI were created conditional on having an average surgeon (defined as having random effects of 0), and multiple comparisons between years were done conditional on surgeon random effect. P values and confidence intervals for multiple comparisons have been corrected for multiplicity based on the approximate distribution of the parameter estimates. All analyses were done using R version 3.2.3 (2015-12-10).
Results
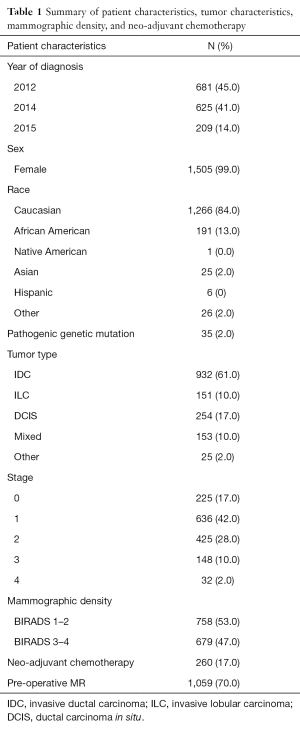
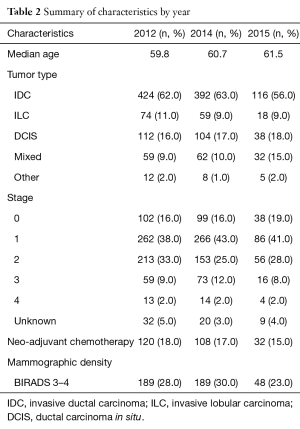
There were a total of 1,515 patients identified who were diagnosed with BC at Cleveland Clinic main campus during the years 2012, 2014, and January to May 2015. Table 1 summarizes patient characteristics, tumor characteristics, and mammographic density. There were 1,491 (98%) patients diagnosed with unilateral BC and 24 (2%) diagnosed with bilateral BC. Two hundred and sixty (17%) of patients received neoadjuvant chemotherapy. Table 2 summarizes characteristics by each year. Invasive ductal carcinoma (IDC) was seen in majority of patients each year, 424/681 (62%) in 2012, 392/625 (63%) in 2014, and in 116/209 (56%) in 2015. Patients in each year were mostly stage I, 262/681 (38%) in 2012, 266/625 (43%) in 2014, and 86/209 (41%) in 2015. One hundred and eighty-nine of 681 (28%) patients in 2012, 189/625 (30%) patients in 2014, and 48/209 (23%) patients in 2015 had mammographically dense breast tissue.
Full table
Full table
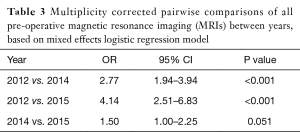
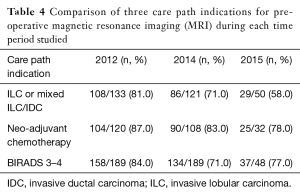
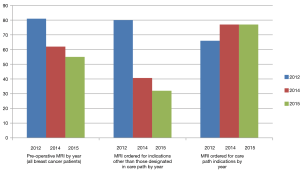
Overall, there were 554/681 (81%) patients receiving a pre-operative MRI in 2012, 389/625 (62%) patients in 2014, and 113/209 (54%) in 2015. Table 3 shows pairwise comparison of all pre-operative MRI performed between years. More patients had a pre-operative MR in 2012 than in 2014 (OR: 2.77; P<0.001; 95% CI: 1.94–3.94) and in 2015 (OR: 4.14; P<0.001, 95% CI: 2.51–6.83). When comparing with patients in 2012, the odds of having a non-indicated pre-operative MRI decreased by 0.18 in patients from 2014 (P<0.001) and by 0.13 in patients from 2015 (P<0.001). Table 4 summarizes three care path indications of patients in each year who received a pre-operative MRI. The majority of patients who had ILC or mixed ILC/IDC, neo-adjuvant chemotherapy, or dense breasts received a pre-op MRI during each year studied.
Full table
Full table
When evaluating the patients without a care path indication for consideration of MRI [indications include: ILC, dense breast tissue, neo-adjuvant chemotherapy, and those with an increased risk of bilateral BC such as those with a genetic mutation (see methods section)], there were 241 patients in 2012, 216 patients in 2014, and 83 patients in 2015. Of these patients, 190/241 (80%) received a pre-operative MRI in 2012, 88/216 (41%) in 2014, and 27/83 (32%) in 2015 (Figure 1). When comparing with patients in 2012, the odds of having a non-indicated pre-operative MRI decreased by 0.18 in patients from 2014 (P<0.001) and by 0.13 in patients from 2015 (P<0.001). In patients who did not have a care paths indication but had an MRI, there were only 23 (12%) patients in 2012, 17 (19%) patients in 2014, and 4 (15%) patients in 2015 that were under the age of 50. It was not possible to determine whether MRIs not indicated by the care paths were patient or provider driven.
Discussion
This study suggests potential benefits of implementing a BC care path to encourage value based standard practice. The implementation of the BC care path was associated with a significant decrease in the total number of pre-operative MRIs and a significant decrease in the number of pre-operative MRIs completed in patients who did not have a BC care path indication.
It is clearly necessary for health care organizations to focus on providing value-based care and sustainable health systems using new models of care delivery to make care more accessible, less costly, and more effective (21). Our institution implemented a BC care path providing information derived from expert consensus and available evidence to assist providers in evaluating and treating BC. A goal of including recommendations for pre-operative MRI in the BC care path was to optimize MRI utilization among our own physicians. This study adds to the knowledge base of the impact of an easily accessible institution-wide care path on value based BC care delivery. The reduction in utilization of MRI and associated reduction in the cost of providing care is the outcome that was demonstrated with this intervention.
Although there has been controversy in the role of routine use of pre-operative breast MRI, there are studies to suggest benefit in the selective use of MRI. The imaging evaluation of ILC is known to be more challenging than other types of BC because of a unique pathological growth pattern (14,15). Contralateral BC has been shown to increase in those with ILC and breast MRI should be considered (14,15). Women with dense breasts also pose a challenge because the sensitivity of mammography is significantly decreased and focused breast ultrasound can miss extensive and multicentric disease (5,16). MRI is used in planning for partial breast irradiation because these patients will not receive sterilizing effect of whole breast irradiation (17). Patients with isolated axillary presentation and unknown primary origin pose a unique challenge. Previously “blind mastectomy” and axillary nodal dissection has been standard of treatment, but that has been challenged since one-third of mastectomy specimens revealed no primary tumor (18). MRI may identify breast tumors not apparent on mammography, but if negative, may reduce the rates of unnecessary mastectomies in these patients. Patients with genetic mutations associated with increased risk of BC should undergo breast MRI for treatment planning. These patients have an increased risk of contralateral BC, which would affect surgical planning. Lastly, breast MRI has been shown to have better accuracy in detecting tumor response after NAC than conventional imaging (20).
In our study, we found no significant difference in the utilization of breast MRI before and after the BC care path in patients with ILC, dense breasts, genetic mutations, and in those who underwent neo-adjuvant chemotherapy. The difference in MRI utilization was seen in patients not having standard indications mentioned in the BC care path before and after implementation. Still, there were a large number of patients receiving MRI after implementation of the care path. We found that young age did not explain for the number of MRIs ordered in patients without a BC care path indication. Investigation into individual provider practice patterns and patient preferences may help to explain pre-operative MRI use and provide insight into how further reduction can be achieved.
Limitations to our study include the retrospective nature of the data collection. This limited the availability of data on patients who had initial work up at an outside institution or who were not included in our tumor registry. Another limitation is that patients who may have had MRI as part of the work up prior to partial breast irradiation or those with axillary presentation were not separately identified. The numbers of these patients were not likely to alter the findings of this study. Lastly, we were not able to evaluate the patients precluded from MRI due to contraindications such as metal, intravenous contrast allergy, body habitus, and claustrophobia.
The BC care path has been instrumental in guiding providers in the evaluation and treatment of BC. We have shown an effective reduction in the utilization of breast MRI, particularly for patients without recommended diagnoses, associated with the care path implementation. We are currently implementing automated systems in the electronic medical record to track and report MRI utilization in real time with the hopes of further optimizing practice patterns.
Acknowledgements
The authors thank Ashley B. Simpson, DO; Najaah Hussain, BS; Yitian Liu, MD; Colin O’Rourke, MS; Stephanie A. Valente, DO; and Joseph P. Crowe, MD.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of the Cleveland Clinic (No. 15-194) and written informed consent was obtained from all patients.
References
- Grobmyer SR, Mortellaro VE, Marshall J, et al. Is there a role for routine use of MRI in selection of patients for breast-conserving cancer therapy? J Am Coll Surg 2008;206:1045-50; discussion 1050-2. [Crossref] [PubMed]
- Vaidya JS, Vyas JJ, Chinoy RF, et al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820-4. [Crossref] [PubMed]
- Lagios MD. Multicentricity of breast carcinoma demonstrated by routine correlated serial subgross and radiographic examination. Cancer 1977;40:1726-34. [Crossref] [PubMed]
- Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985;56:979-90. [Crossref] [PubMed]
- Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in Fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol 2004;183:1149-57. [Crossref] [PubMed]
- Crowe JP, Patrick RJ, Rim A. The importance of preoperative breast MRI for patients newly diagnosed with breast cancer. Breast J 2009;15:52-60. [Crossref] [PubMed]
- Slanetz PJ, Edmister WB, Yeh ED, et al. Occult contralateral breast carcinoma incidentally detected by breast magnetic resonance imaging. Breast J 2002;8:145-8. [Crossref] [PubMed]
- Lehman CD, Blume JD, Thickman D, et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: results from the International Breast Magnetic Resonance Consortium (IBMC) trial. J Surg Oncol 2005;92:9-15; discussion 15-6. [Crossref] [PubMed]
- Braun M, Pölcher M, Schrading S, et al. Influence of preoperative MRI on the surgical management of patients with operable breast cancer. Breast Cancer Res Treat 2008;111:179-87. [Crossref] [PubMed]
- Wallace AM, Daniel BL, Jeffrey SS, et al. Rates of reexcision for breast cancer after magnetic resonance imaging-guided bracket wire localization. J Am Coll Surg 2005;200:527-37. [Crossref] [PubMed]
- Fischer U, Zachariae O, Baum F, et al. The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol 2004;14:1725-31. [Crossref] [PubMed]
- Morrow M. Magnetic resonance imaging in breast cancer: one step forward, two steps back? JAMA 2004;292:2779-80. [Crossref] [PubMed]
- Solin LJ. Counterview: Pre-operative breast MRI (magnetic resonance imaging) is not recommended for all patients with newly diagnosed breast cancer. Breast 2010;19:7-9. [Crossref] [PubMed]
- Mann RM, Veltman J, Barentsz JO, et al. The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol 2008;34:135-42. [Crossref] [PubMed]
- Mann RM, Hoogeveen YL, Blickman JG, et al. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat 2008;107:1-14. [Crossref] [PubMed]
- Debald M, Abramian A, Nemes L, et al. Who may benefit from preoperative breast MRI? A single-center analysis of 1102 consecutive patients with primary breast cancer. Breast Cancer Res Treat 2015;153:531-7. [Crossref] [PubMed]
- Godinez J, Gombos EC, Chikarmane SA, et al. Breast MRI in the evaluation of eligibility for accelerated partial breast irradiation. AJR Am J Roentgenol 2008;191:272-7. [Crossref] [PubMed]
- Fortunato L, Sorrento JJ, Golub RA, et al. Occult breast cancer. A case report and review of the literature. N Y State J Med 1992;92:555-7. [PubMed]
- Sardanelli F. Overview of the role of pre-operative breast MRI in the absence of evidence on patient outcomes. Breast 2010;19:3-6. [Crossref] [PubMed]
- Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology 2012;263:663-72. [Crossref] [PubMed]
- Porter ME. Value-based health care delivery. Ann Surg 2008;248:503-9. [PubMed]
- Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med 2003;138:288-98. [Crossref] [PubMed]


