Breast reconstruction with absorbable mesh sling: dynamic infrared thermography of skin envelope
Introduction
In 2013, Japanese public health insurance began to pay for prosthesis-based breast reconstruction. Commonly, to reconstruct a breast mound with an implant, a skin flap must be preserved that is wide enough and broad enough to completely cover the surface of the implant. A muscle also must be covered with an implant. The skin flap should not only be wide enough to cover the entire implant, but it should also be healthy, with a good blood supply. Covering the implant with muscle is also important to prevent flap necrosis, which could cause implant exposure. So implants have been placed in a submuscular space, and the subpectoral space is typically used in such implant-based procedures, including breast augmentation.
At the case of immediate reconstruction, these are two big matters. First, total mastectomy requires the removal of a large ellipse of skin that includes the nipple-areolar complex (NAC), so it is too tight to reconstruct breast immediately. Therefore, in 1991, skin sparing mastectomy (SSM) was first performed by Toth and Lappert (1). Also, in same year, Kroll reported on their experience with 100 breast cancer patients undergoing SSM and immediate reconstruction (2). Moreover, nipple-sparing mastectomy (NSM) technique was reported. It is similar to SSM, but spares the NAC, and hence (3), has an advantage over SSM in completely preserving the breast skin. The first matter was resolved by this technique. Second, in NSM followed implant-based reconstruction, it is difficult to make a complete muscle pocket (combined the pectoralis major muscle and the serratus anterior muscle), especially in the case of ptosis breast. So, the one-stage reconstruction method should be abandoned, and instead a tissue expander should be used to stretch the muscle-envelope, even if enough of the skin flap was conserved. To address this problem, Breuing reported a new procedure using an acellular cryopreserved dermal matrix [AlloDerm®: bioprosthetic material manufactured from human cadaveric or animal sources (4)] sling to reestablish the lower pole of the pectoralis major muscle (5). For this technique to be effective, a subpectoral-sub-AlloDerm pocket could be created that completely encloses the breast implant. Then, by tailoring the width of the AlloDerm, the degree of lower-pole fullness can be adjusted. Indeed, acellular dermal matrices have allowed greater pocket control (6,7), and have improved cosmetic outcomes (8). Unfortunately, this material is very expensive and is not available Japan. As a substitute for AlloDerm, Tessler used polyglactin 910 (Vicryl; Ethicon Inc., USA) mesh for an inferior-lateral sling in one-stage, prosthetic-based reconstruction and reported excellent results (9). However, Vicryl mesh is resorbed three to 4 weeks after implantation, consequently losing function as a supporting structure. There remain strong doubts about the stability of the blood supply to the inferior part of the flap when it is in direct contact with inner implant.
To assess the safety of the skin flap perfusion of this absorbable mesh sling (AMS) procedure, we performed thermography to measure flap temperature using dynamic infrared thermography (DIRT) technique (10,11). DIRT is based on the relationship between dermal perfusion and the rate of change in skin surface temperature following transient thermal changes (12,13).
In this study, we examine the complications and the results of DIRT in 40 patients, 1 year after undergoing breast reconstruction with the AMS procedure. And we consider the safety of the skin flap of this procedure.
Methods
Patients
We have performed implant based breast reconstruction in 202 patients since October 2010. In April 2014, we began using a Vicryl mesh sling to anchor the pectoralis major muscle to the chest wall. We have already performed the procedure on 80 patients (81 breasts) who underwent NSM, 73 of which, underwent immediate, one-stage breast reconstruction. After obtaining informed consent by each patients and approval from the institutional ethics board of the Japanese Red-Cross Society of Himeji, we conducted a retrospective review of all breast implant based reconstruction procedures.
After consensus was reached through discussion in our breast surgery team, oncologists, board certified in their specialty, made the final determination about patient selection, indications for mastectomy technique, and the ability to immediately perform expander or implant based breast reconstruction. Candidates for the NSM procedure were carefully selected according to the NCCN practitioner’s guidelines: “patients with early-stage, biologically favorable breast cancer that is peripherally located in the breast” (14).
Also in cases that the NAC had to be removed, an effort was made to perform a one-stage reconstruction using an applicable cohesive gel when possible. If the flap became too tight, and was insufficient to cover the implant, the reconstruction-procedure was converted from a one-stage into a two-stage procedure using a tissue expander (5 cases in 80 cases, Table 1). Then, within 1 month to 2 months, we would inflate the expander, and enlarge the pocket enough to implant the desired cohesive gel. All procedures were completed before the next scheduled radiotherapy.

Full table
Surgical technique
The operations were performed under general anesthesia. On a case-by-case basis, sentinel lymph node biopsy or axillary lymph node dissection was performed (Table 1). Surgery of the axilla did not influence the indication for, or the type of breast reconstruction procedure, aside from the skin incision. The surgeon made an incision in the skin on the inframammary line or the line around the nipple-areolar circle, and extending it to the axilla.
First, the breast was resected the layer of superficial fascia. After reaching the nipple, the sample specimen was removed for intraoperative pathological examination by frozen section. If the sample specimen showed signs of malignant cancer, the procedure was changed from NSM to SSM, and the NAC was resected. Including the nipple, if the immediate pathological examination revealed malignancy in the flap, e.g., at the site above the tumor, the skin resection was widened. If the area of the flap was of insufficient size to cover the entire implant, the procedure was converted from a one-stage reconstruction procedure to a two-stage procedure.
After completion of NSM, the reconstructive technique was begun with the elevation of a subpectoral pocket. Detachment of the pectoralis major muscle from the sternum, for example for the right breast, proceeded to the 6- and 10-o’clock levels, with adjustments made as necessary to accommodate the desired implant (Figure 1A).
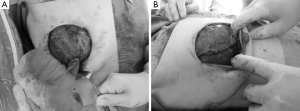
In principle, at least one saline sizer, the best cohesive gel from the preselected candidates, was then chosen based on mastectomy weight and measurements of the patient’s breast base diameter. The sizer was then placed into the partial subpectoral pocket and filled with normal saline to the desired volume. A marking pen was then used to trace the projected outline of the implant to assist tacking of the Vicryl knitted mesh in the proper position.
The knitted Vicryl mesh was then sutured into place using 4-0 Vicryl sutures, ensuring proper lateral and inferior fold placement. After securing the inferior and lateral mammary folds, the sizers were placed into the pocket. The skin flaps were stapled closed; and the patient was placed in a seated position to observe symmetry, contour, and proper fold placement. The patient was then returned to the supine position. The final implant was chosen and placed in the pocket, and the Vicryl mesh was secured to the pectoralis muscle with Vicryl 4-0 sutures (Figure 1B). Suction drains were placed through a tunnel of subcutaneous tissue and placed in the suprapectoral and subcutaneous spaces. Adjustments were made as necessary, hemostasis was ensured, and the wound was then closed.
Assessment of complications
We examined the outcomes for 80 patients undergoing this AMS procedure, and safety was assessed in 40 patients 1 year after the procedure was performed. Complications were divided into minor complications, major complications requiring surgical intervention, and major complications requiring the reconstruction procedure to be halted, and examined according to onset in the post-operative period.
Assessment of perfusion in skin flap and DIRT
The perfusion of skin flaps on reconstructed breast mounds was examined in terms of the appearance of a low-perfusion area by DIRT. A FLIR (FLIA T340, FLIR Systems Inc., 27700 SW Parkway Avenue, Wilsonville, OR 97070, USA. Email: webmaster@flir.com) infrared camera was used to monitor skin surface temperatures. An accuracy of 0.1 °C in measurements was obtained. Infrared thermal images were taken at regular intervals to analyze the rate and the pattern of rewarming. First, a picture was taken and thermography was performed on the breast, before cooling stress was applied, in the upright position. Next, the candidate was placed in the supine position and both breast mounds were covered with ice-water packs. The chest wall was cooled broadly and uniformly for 10 seconds. Then the patient was placed in the standing position to perform dermal thermography and monitor the changes in dermal temperature, immediately, 5, and 10 minutes after cooling.
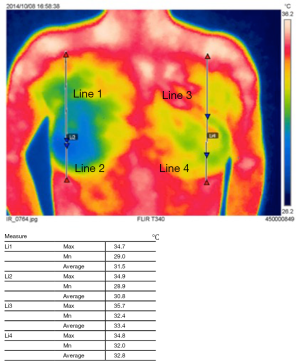
Each thermographic scans were analyzed using the FLIR software (FLIR Tool®, FLIR System). Temperature data were acquired along two lines, from the nipple to the center of the clavicle (superior line: s-line), and from the nipple to the inframammary line (inferior line: i-line). The software calculated the following data: maximum, minimum, and average temperature on each line (Figure 2). Average temperatures were determined from the lines of the thermographs at the following points: initial (before cooling) and, immediately, 5, and 10 minutes after cooling and, from ipsilateral and contralateral breast mounds.
From the initial thermography, uniformity was assessed on each side and the difference between the upper and lower sides, and the symmetric property between the ipsilateral and contralateral sides.
When performing DIRT, we could not cool the flap uniformly in every patient. Therefore, a linear graph of the rewarming pattern was drawn using repeated measurements and compared by two-way ANOVA.
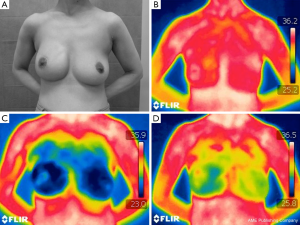
In Figure 3A, a case of breast ptosis is shown 1 year after NSM and one-stage reconstruction using the AMS procedure, on the right side breast, and the patient did not undergo adjuvant radiotherapy. The cosmetic outcome was excellent, including good flap condition. Before cooling, the skin temperature was almost symmetric, and no cold spot was observed (Figure 3B). Once both breasts were cooled using an ice-water bag (Figure 3C), and the rewarming pattern was assessed. The difference in rewarming speed, after removal of the cooling stress, between the ipsilateral (right) and contralateral (left) sides, and between the superior and inferior areas in the reconstructed mound are shown in the Figure 3D.
Statistical analysis
Initial skin temperature at each site of both breasts (s-lines and i-lines on ipsilateral and contralateral breast) were analyzed with the Student’s t-test (two-tailed), and normally distributed data are presented as mean ± SD. We performed repeated measurements of skin temperatures in the s-lines and i-lines at three time points (immediately, 5, and 10 minutes after cooling), and data summaries of two groups (e.g., the i-line on the ipsilateral side and the i-line on the contralateral side) were compared using two-way-repeated measures ANOVA. Mauchly’s test of sphericity was used to evaluate whether the sphericity assumption was violated. Comparisons were considered significant if P<0.05. Statistical analysis was conducted with SPSS statistics, version 22, resource 22.0 for Windows.
Results
In our center, 80 patients (81 breasts) underwent breast reconstruction using the AMS technique, and 1 year has passed since 40 of these patients underwent the procedure (Table 1). In 73 cases, reconstruction was performed immediately after surgery for breast cancer and in one-stage. Using the AMS technique, the ratio of implant volume to specimen weight was 1.41 on average (range, 0.78–2.63), for patients who underwent immediate, one-stage reconstruction.
Complication outcomes are presented in Table 2. Four cases with minor flap necrosis were treated with sharp debridement in the office. Of the 61 nipple-sparing mastectomies, two nipples were affected by partial necrosis, but did not require surgery.
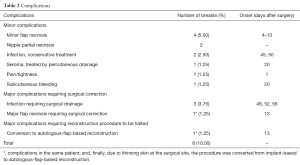
Full table
Major complications occurred in 4 cases, requiring surgical correction (5%): staphylococcus aureus was isolated from implant pockets. Surgical-site infection occurred in 3 cases (3.75%). In all three cases, axillary dissection had been performed, and infection was diagnosed and confirmed more than 6 weeks after surgery (48, 52, 56 days: primary causative organism was staphylococcus aureus in all cases).
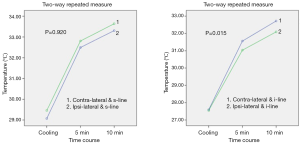
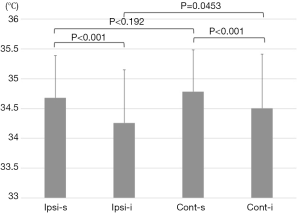
In skin-flap thermography, we compared the surface temperatures in four areas: the superior and inferior areas on the ipsilateral side, and the same on the contralateral side. The average of the line (the “s-line” was drawn from the nipple to the center of the clavicle, and the “i-line”, to the center of the infra-mammary line, Figure 2) was used as a reference for the temperature of each area. In the initial condition (before cooling stress), the temperature was lowest in the inferior area on the ipsilateral side [ipsil-inferior (Ipsi-i) 34.26±0.71, ipsi-superior (Ipsi-s) 34.36±0.89, contra-superior (Cont-s) 34.78±0.70, contra-inferior (Cont-i) 34.50±0.91, Figure 4]. The inferior side was significantly cooler than the superior side, even in the healthy side of the breast mound. A comparison of the ipsilateral and contralateral sides revealed a significant difference between the inferior sides (P=0.0453), but not the superior sides (P=0.192).
Two-factor repeated measures ANOVA (within-subject factor and interaction) was performed to analyze the effect of measurement site (ipsilateral or contralateral side), as the independent factor, on rewarming patterns. This analysis was performed to determine relationships between the groups (ipsilateral or contralateral side) and over time, using F test. (The time course was as within-subject factors; immediately, 5, and 10 minutes after cooling: the groups, ipsilateral or contralateral side, were as between-subjects factors: Mauchly’s test of sphericity, P=0.500). There was a significant correlation between the time course of rewarming patterns and the ipsilateral or contralateral inferior sites (time course × ipsilateral or contralateral side, df =2, MS =1.900, F=4.456, P=0.015, Figure 5). However, there was no significant difference in superior sites (df =2, MS =0.029, F=0.083, P=0.920).
Discussion
As expected, the skin temperature on the inferior side of the reconstructed mound was cooler than on the healthy side; and, DIRT showed that blood perfusion was comparably poor on the inferior area of the AMS-reconstructed breast mound where the pectoralis muscle could not line the inside of the pocket. However, there have been no complications reported more than 2 months after surgery. Also, breast mounds reconstructed with AMS did not exhibit ischemic changes, and so far, there has been no need to halt reconstruction of a breast mound while using the AMS procedure. One concern of this study is whether poor perfusion of the skin flap, which was observed in the inferior area of breast mounds reconstructed with AMS, would be an indicator of poor prognosis. After the initial favorable time for infection, the breast mounds reconstructed with AMS appear completely complication free 1 year after the procedure was performed.
When comparing the AMS procedure with any other methods, in this regard, however, the overwhelming advantage offered by the AMS procedure should be considered. The design of a prospective study about the feasibility of the AMS procedure should assess not only the cosmetic results, but also categorize them in the immediate, and one-stage reconstruction surgery. In our experience, immediate, one-stage, and prosthesis-based breast reconstruction have been severely limited without using the AMS procedure, because we cannot employ AlloDerm. One-stage reconstruction using autologous tissue (e.g., LDF flap or DIEP flap) is a more invasive surgery and sacrifices the healthy donor site, and thus is an inappropriate comparison. A complete muscle pocket, built for implantation, using pectoralis major and serratus anterior muscles would be impossible for most breasts, especially large breasts with ptosis. We have achieved, on average, an implant volume to specimen weight ratio of 1.41. AMS resolved the imbalance between favorable implant volume and the scant muscles available around the breast. If the serratus anterior muscle was used instead of AMS, we occasionally selected two-stage reconstruction; once the tissue expander was inserted, the flap and muscle pocket were gradually expanded after the initial surgery. Then, the tissue expander was replaced with cohesive gel in the second surgery. Adjuvant radiotherapy is known to complicate the reconstruction process and increase the potential for other complications (15-17).
Given this perspective, if we wish to reconstruct a large breast immediately after surgery for breast cancer in one-stage, our options are extremely limited. The AMS procedure overcomes the limitation of insufficient muscle pocket size for favorable implants. During the first 2 months after reconstruction surgery, when breast mounds are most susceptible to bacterial infections, and for at least 1 year after reconstruction surgery, there have been no ischemic flap complications in breast mounds that we reconstructed with AMS. Based on these findings, we will continue to perform reconstruction using AMS methods. However, DIRT revealed apparently poor perfusion of the skin flap, in the inferior area, where the flap was not lined up with the muscle. In the long term, we should be able to acquire sufficient data regarding flap condition to determine the feasibility of this method.
Our examination has limitations. First, as described above, there was no evidence that poor perfusion in the lower area of AMS-reconstructed breast mounds, as observed during DIRT examination, was indicative of any major complications in the future. Second, we did not compare our DIRT data with data of conventional reconstruction procedures, e.g., latissimus dorsi muscle flap repair or covering the implant with a complete muscle pocket designed from both pectoralis major muscle and serratus anterior muscle.
Conclusions
Blood perfusion was comparably insufficient in the inferior area of the reconstructed breast mound with AMS, where the muscle could not be used to line the inside of the envelope. However, there were no severe flap complications due to ischemia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: We obtained informed consent by each patient and approved by the institutional ethics board of the Japanese Red-Cross Society of Himeji.
References
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991;87:1048-53. [Crossref] [PubMed]
- Kroll SS, Ames F, Singletary SE, et al. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast. Surg Gynecol Obstet 1991;172:17-20. [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [Crossref] [PubMed]
- Davila AA, Seth AK, Wang E, et al. Human Acellular Dermis versus Submuscular Tissue Expander Breast Reconstruction: A Multivariate Analysis of Short-Term Complications. Arch Plast Surg 2013;40:19-27. [Crossref] [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [Crossref] [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-410e. [Crossref] [PubMed]
- Tessler O, Reish RG, Maman DY, et al. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg 2014;133:90e-99e. [Crossref] [PubMed]
- Salmi AM, Tukiainen E, Asko-Seljavaara S. Thermographic mapping of perforators and skin blood flow in the free transverse rectus abdominis musculocutaneous flap. Ann Plast Surg 1995;35:159-64. [Crossref] [PubMed]
- de Weerd L, Miland AO, Mercer JB. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography. Ann Plast Surg 2009;62:42-7. [Crossref] [PubMed]
- Zetterman E, Salmi AM, Suominen S, et al. Effect of cooling and warming on thermographic imaging of the perforating vessels of the abdomen. Eur J Plast Surg 1999;22:58-61. [Crossref]
- Wilson SB, Spence VA. Dynamic thermographic imaging method for quantifying dermal perfusion: potential and limitations. Med Biol Eng Comput 1989;27:496-501. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology Breast cancer. Version 2.2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Cordeiro PG, Albornoz CR, McCormick B, et al. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast Reconstr Surg 2015;135:1509-17. [Crossref] [PubMed]
- Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg 2009;124:395-408. [Crossref] [PubMed]
- Hirsch EM, Seth AK, Dumanian GA, et al. Outcomes of tissue expander/implant breast reconstruction in the setting of prereconstruction radiation. Plast Reconstr Surg 2012;129:354-61. [Crossref] [PubMed]