Evaluation of discrepancies in weights of fresh and fixed specimens in breast surgery: a retrospective cohort study
Introduction
Weight of the resected specimen has significant role in the field of breast surgery. This is particularly important with the paradigm shift towards more conservative surgical approach for cancer and wider popularity and acceptance of symmetrisation procedures performed on the contra lateral side. Moreover, there are strict guidelines to follow regarding the weight of specimen in situations such as open diagnostic biopsies (1). The guidelines suggest recording of fresh tissue weight. However, in many units, the guidance regarding the size or volume of tissue removed is taken from the weight of preserved pathological specimens.
Tissue handling after surgery is less monitored in most of the institutions and its impact on the tissue is not well documented (2). Considerable variation exists across various units, especially in the length of time the specimen is kept in the fixative solution. There is very limited data available in literature regarding the reliability of the specimen weight which is preserved in a fixative solution.
It has been reported that there is significant shrinkage of specimen when preserved in formaldehyde and a number of studies have assessed the effect of specimen handling on final tumour margin (2).
Series by Yeap et al. showed shrinkage of 34% following fixation of specimens and reported that the shrinkage is more observed with wide local excision specimens in comparison with mastectomy specimens (3). The proximity or positive tumour margin can influence not only the need for further surgery, but also other modes of adjuvant treatment such as radiotherapy along with their side effects (4).
There is no uniformity in the duration for which the specimen stays in the preservative solution prior to being prepared for histological examination. This is variable according to the day and time of surgery and the frequency of collection of specimen in various units. Calculation of weight in the pathology department can be influenced by the amount of fixative retained by the specimen while weighing. There is also evidence for various physical and chemical changes to the specimens induced by fixative solution. All these factors can lead to calculation of spurious weights. Any surgery or future plans based on this weight may thus be misleading.
It has been shown in animal studies that commonly used fixatives such as formalin affect the tissues’ physical and chemical characteristics (5,6). Biochemical characteristics of the tissues preserved, type, strength and volume of preservative used and the length of time the specimen is stored in fixative, could all influence the weight of the specimen (5). The study by Fraser et al. showed in their series that the maximum amount of changes in formalin occurred during the first 24 hours (5).
There is also suggestion in the literature that in case of dense breasts, specimen weight tends to overestimate the volume (7). There is little in the literature looking at the difference in specimen weights with fixatives in dense and fatty breasts. This again is significant in oncoplastic breast surgery as accurate measurements are important in planning the future reconstructive procedures (7,8).
Aim of this study was to assess the changes in weight of specimen with preservation.
Methods
All patients who underwent breast surgery in a single district general hospital from a period between January 2013 and June 2015 were included. Patients with normal (as in symmetrisation procedures), benign and cancer histology were considered.
Primary endpoint of this study was to quantitatively evaluate the discrepancies in actual weight of fresh specimen in breast surgery and the weight of specimen after being kept in a fixative solution for a variable length of time. Secondary endpoints were as follows:
- To assess the correlation of age and the variability of weights after being kept in a fixative solution;
- To evaluate the impact of body mass index (BMI) on the specimen weights;
- To assess the effect of breast density on the variability of specimen weights;
- To evaluate the impact of length of preservation on weight of specimens;
- To assess the impact of initial weight on variation while preserved in fixative solution;
- To assess the impact of inclusion of overlying skin in the breast specimen on variation in weight when preserved in fixative solution.
Exclusion criteria
Patients who had undergone breast surgery during this period with no documentation of fresh specimen weights in the operative notes.
The study was carried out retrospectively and was approved by local clinical governance department (the United Lincolnshire Hospitals NHS Trust Clinical Governance Department approval number is P2526). All patients were females who underwent breast surgery for a range of pathology both in the diagnostic and therapeutic setting. Fresh specimen weight was calculated in the operating theatre immediately after excision. Subsequently, specimens were collected and transported to the pathology department at frequent intervals. Specimens were kept in the fixative solution for a variable length of time which was dependant on the time of day when surgery was carried out.
Data collection
Demographic details, data on BMI, age, date of surgery, time of surgery, time of processing the specimen in the pathology department and final histology were collected retrospectively from the case notes, operation room management information system (ORMIS) and histopathology reports.
As it is not a common practice to record the breast density in mammogram reports, principal investigator along with a Consultant Radiologist reviewed the mammograms of patients included in the study and categorised the breast density as per Breast Imaging Reporting And Data System (BI-RADS) guidelines (9).
Data analysis
The data was anonymised and saved in a password protected secure file with access limited to the principal investigator. Statistical analysis was carried out with SPSS software (IBM SPSS statistics for Windows, Version 22.0, Armonk, NY, IBM Corp., USA).
Non Parametric tests were used to compare the groups and scatter plots were generated for correlating variables. A multivariate linear regression analysis was carried out with different variables to assess the factors which influenced the difference in weight of the specimens. All the statistical tests were considered to be significant at a P value of <0.05.
Results
A total of 229 patients were included in the study. The median age was 63 [interquartile range (IQR) 51–73] years. The median BMI was 27.9 (IQR 24.3–31.75) kg/m2. Majority of the patients had a mammographic breast density of BI-RADS category 2 (N=104, 46%). Fifteen percent (N=34), 25% (N=56) and 14% (N=32) patients had BI-RADS score of 1, 3 and 4 respectively.
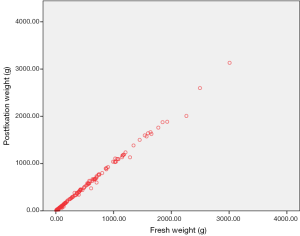
Median weight of the fresh specimens was 104 (IQR 44–535) g. Median weight of specimen after kept in the fixative solution was 99 (IQR 43–525) g. This difference in weight between the two groups was statistically significant (Figures 1 and 2, P value=0.000, Wilcoxon Signed Rank test).
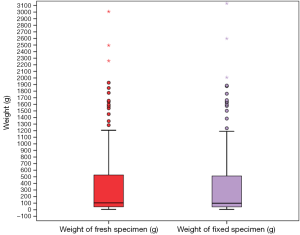
The mean difference in weight between fresh and fixed specimens was 12.41 (range, 0–252) g. The variation in weight was not unidirectional. Seventy specimens weighed less when preserved and in 120 patients, post fixation specimen weight was higher. Thirty nine patients showed similar fresh and fixed specimen weights.
Age and specimen weights
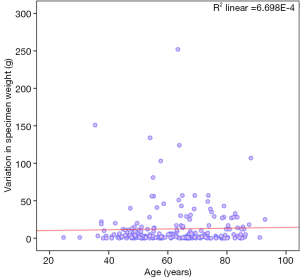
Figure 3 shows the correlation of age with variation in specimen weight and did not show any significant correlation (Spearman’s rho 0.051; P value =0.440).
Relationship between duration of preservation and variation in specimen weight
The median duration of preservation of specimens was only 15 (IQR 2.58–17.25) hours.
There was no significant correlation between the variation in weight and the duration of specimen kept in the fixative solution prior to processing (Spearman’s rho 0.105; P value =0.114).
Further subset analysis by dividing the patient population to two subgroups dependant on the duration of preservation (<15 and >15 hours) also failed to show any significant difference in variation of specimen weights (P value =0.135, Mann-Whitney U test).
Specimen weight and BMI
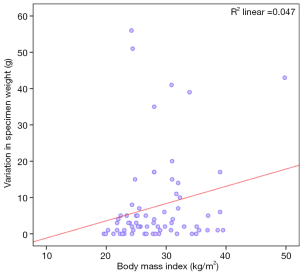
The study failed to show any significant correlation between the BMI of patients and the changes in specimen weight with fixative solution (Figure 4: Spearman’s rho 0.223; P value =0.06).
Breast density and specimen weight
There was no significant difference in variation of specimen weight in different BI-RADS breast density categories (P value =0.725, Kruskal Wallis test).
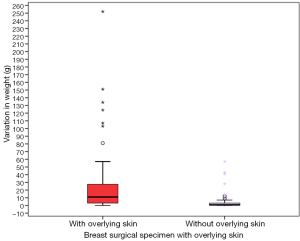
Influence of inclusion of overlying skin in breast specimen and variation of weight with preservation
Comparison between variation in weights of specimens with [mean 22.53 (range, 0–252) g] and without overlying skin [mean 3.32 (range, 0–57) g] showed a significant difference [Figure 5 (P value =0.000, Mann-Whitney U test)]. However on multivariate regression analysis this observation failed to show any significance (P value =0.384).
Relationship between initial weight of specimen and variation with preservation
Specimens which weighed more than 1,000 g as fresh specimens showed larger variation after being kept in the fixative solution (P value =0.000, Mann-Whitney U test).
Variation was significantly pronounced when the initial specimen weights were higher (Figure 6, Spearman’s rho 0.707; P value =0.000).
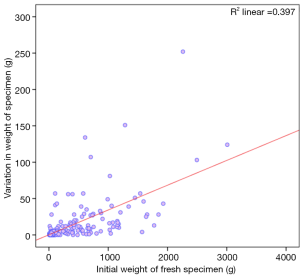
On subset analysis, this variance in specimen weight was consistent when the patient group was limited to those with an initial weight of less than 1,000 g (Spearman’s rho 0.622; P value =0.000) as well as those with initial weight less than 500 g (Spearman’s rho 0.525; P value =0.000). As most of the breast conservation surgical specimens are smaller, the analysis was further repeated in the subset of patients with an initial specimen weight of less than 150 g (Spearman’s rho 0.357; P value =0.00) which again showed a consistent trend. On Multivariate analysis this was the only significant variable which influenced the variation in weight with preservation (P value =0.000).
Variation in weights in patients who had diagnostic open biopsy
As there are strict breast screening guidelines restricting the recommended weights of open diagnostic biopsy specimens to 20 g limit, the variation in weight was assessed in this group. However this subset had only a limited number of patients (N=15) and the tests failed to reveal any significant difference.
Median weight of fresh specimen was 13 (IQR 9–16) g and that of fixed specimen was 13 (IQR 9–19) g. P value =0.667; Wilcoxon Signed Rank test.
Table 1 Summarizes the correlation of variables to the variation in weight.
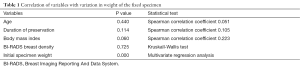
Full table
Discussion
To achieve good symmetry and aesthetic results with breast surgical interventions both in the cosmetic setting as well as in the oncoplastic setting after cancer treatment, it is very important to assess the volume of breast accurately. There are numerous techniques in use for assessing breast volume in the pre-operative setting such as anthropometric measurements, 2 D images such as mammogram and ultrasound scan, water displacement technique, using 3 dimensional (3 D) negative casts of breasts with plaster or thermoplastic materials, Computed Tomography (CT) or magnetic resonance imaging (MRI) assisted 3 D modelling of breast and 3 D body surface imaging (10-20). However, most of these techniques are cumbersome and expensive and for practical purpose, most of the units use the weight of resection specimen as a rough guide for breast volume measurements. Parmar et al. in their small series compared the weight of breast specimen with volume measured by water displacement technique and they found no statistical difference between the use of measurement by weight or volume (19). This observation was same when compared in pre and post-menopausal women and as well in women with different breast densities (19). Previous studies suggest that the specific gravity of water is 1.00 and that of fat is 0.92, hence it is acceptable to assume the weight of breast tissue (a mixture of glandular tissue and fat) equals volume as overall specimen density approaches 1.0 g/cm3 (8,19).
Hence, specimen weight is an important parameter in the current era of wider practice of oncoplastic breast conservative procedures and breast. The commonly used fixative solution in surgical practice is formaldehyde which preserves the tissue from degradation and maintains cell structure facilitating histopathological examination. The fixative of choice in most centres worldwide for light microscopy is 10% neutral buffered formalin (4% formaldehyde) (21). The main action of formaldehyde is to cross link amino groups in proteins through the formation of methylene bridges (22). There is evidence from previous series that the specimen does undergo various morphological changes with the preservative solution (5,6,23-26). Lukacs et al. showed that human radical prostatectomy specimen undergo weight reduction after preserving in the fixative solution (22). However, to the best of the author’s knowledge, there is very limited published data available on the effect of fixative solutions on weight of breast resection specimens (27).
In our series, there was statistically significant change with weights of the specimen after being kept in a fixative solution. Interestingly, these changes were not unidirectional. Some of the specimens showed a decrease (N=70), while others showed either no change (N=39) or an increase (N=120) in weight of fixed specimens in comparison with actual fresh specimen weight. This observation is in contrast to Krekel et al. who compared pre and post fixation specimen weights and volume in a small number of lumpectomy patients and failed to observe any significant difference (27). However our study population was different and we have included wider spectra of patients.
Fraser et al. postulated that the shrinkage of tissue brought out by cross linking of protein molecules could lead to reduction in weight. However this process does take prolonged time to complete and on most occasions, the specimens were not kept in the fixative solutions for a very long period of time. The median duration in our series was only 15 (IQR 2.58 to 17.25) hours.
Some of the weight loss for peri renal fat in animal studies was attributed to rupture of cell membrane and subsequent release of cellular contents (5,28). It is difficult to decipher the precise mechanism contributing to reduction in weights of the specimen after fixation in breast surgery.
Similarly, there could be multiple factors contributing to the increase in weight of fixed specimens. One of the factors suggested from animal studies was the osmotic pressure and tonicity of tissue fluid, fixative solution and membrane permeability (5,29). Another possible common factor which influences the changes in weight is variation in the amount of fixative solution removed before weighing in the pathology department (5). Fraser et al. observed that most of the weight changes occurred in the first 24 hours and the variability was more pronounced with peri renal fat in their animal study series.
In our series, there was no statistically significant correlation observed between the variation in specimen weight and BMI or breast density. Even though there was a trend towards increased variation with higher BMI, this observation failed to achieve any statistical significance (P value =0.06).
It is interesting to note that the variation was significantly pronounced on initial observation with breast surgical specimens which included overlying skin. Even though this observation failed to retain significance on multivariate regression analysis, this is important for practical purposes, as most of the mastectomy and therapeutic mammoplasty tissue will have overlying skin in the resected specimens. These patients are usually candidates for delayed reconstruction or contra lateral symmetrisation procedures in future and hence accurate assessment of weight and volume of resected specimens is vital to achieve good aesthetic outcome.
In this series, there was significantly pronounced variation in weight of specimens which were heavier on initial weighing. Larger specimens may retain more fixative solution and the variability in removal of surface fluid before weighing in the pathology department could be a possible factor contributing to this observation.
The difference in observation in our series and the study by Krekel et al. could be due to multiple factors. Firstly, they included only lumpectomy specimens with a mean weight of 47.7 g and our series included a broader range of surgical procedures such as mastectomies, therapeutic mammoplasties and breast reduction procedures. We feel that inclusion of these subsets of patients were important as measurement of resection specimen weight and volume is more vital in this group of patients who are likely to undergo further reconstruction or symmetrisation procedures. The two factors which showed significant association with variation in fixed specimen weights in our series were larger and heavier specimens and inclusion of overlying skin in the resection tissue. Both these subsets of patients were not included in Krekel et al. series (27). It is also important to note that the mean duration between measurements of weight of fresh and fixed specimen were significantly shorter in Krekel et al. series (3.48 hours).
There was no statistically significant correlation between the duration of specimen being kept in the fixative solution and the variation in weight. This observation was similar to that of study by Krekel et al. even though the duration of preservation of specimens in fixative solution in this series was longer (27).
Interestingly, open biopsy specimens did not show any statistical difference between weights of fresh and fixed tissue. However it is worth noting that this was a small group with a limited number of patients.
The major limitations of the study were as follows. Firstly, the study was conducted retrospectively. Not all consecutive patients were included during the study period, as those patients without details of fresh specimen weights documented in the operation notes were excluded. Secondly, the data on volume of fixative solution used to transport the specimens were not available. Therefore, it is difficult to comment whether this would have had any effect on the weight of the specimens. Thirdly, calculation of weights in operation theatre and pathology departments was not standardised. The amount skin resected along with breast specimen was not quantified. Another important limitation of our study was lack of measurement of volume of specimens. Lastly, subset analysis for assessing variation in weights in the subgroup that underwent open diagnostic biopsies was with a small number of patients. This may induce type 2 error in statistical analysis in this group.
Recommendation would be to conduct a prospective study evaluating the actual weight of fresh specimen with weight of fixed specimen with a larger number of patients. This should also be compared with the measurement of volume of resected specimen after standardisation of methods of measurements. It would be interesting to find out the changes in weight after standardisation of the volume of fixative solution used along with uniform specimen transport protocol and the duration of preservation.
Conclusions
There is significant variation in the measurement of weights of breast specimen as fresh immediately after excision and after being preserved in a fixative solution for a length of time. The amount of variation in specimen weight is not uniform and is more pronounced with heavier specimens. Age, BMI, breast density or duration of specimens in the fixative solution did not influence variation in weights of specimens preserved in fixative solution. It is difficult to assess importance of this degree difference weight in the routine clinical practice and needs further validation with a larger prospective study.
Acknowledgements
None.
Footnote
Conflicts of Interest: Author has used the data from this work in the dissertation for Master’s degree in Oncoplastic Breast Surgery from University of East Anglia, United Kingdom.
Ethical Statement: The study was carried out retrospectively and was approved by local clinical governance department (the United Lincolnshire Hospitals NHS Trust Clinical Governance Department approval ID number is P2526).
References
- Sibbering M, Watkins R, Winstanley J, et al. Quality Assurance Guidelines for Surgeons in Breast Cancer Screening. Fourth edition. Sheffield: NHS Cancer Screening Programmes, 2009.
- Dooley WC, Parker J. Understanding the mechanisms creating false positive lumpectomy margins. Am J Surg 2005;190:606-8. [Crossref] [PubMed]
- Yeap BH, Muniandy S, Lee SK, et al. Specimen shrinkage and its influence on margin assessment in breast cancer. Asian J Surg 2007;30:183-7. [Crossref] [PubMed]
- Vallis KA, Pintilie M, Chong N, et al. Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol 2002;20:1036-42. [Crossref] [PubMed]
- Fraser KW. Effect of storage in formalin on organ weights of rabbits. New Zealand Journal of Zoology 2012;12:169-74. [Crossref]
- Parker RR. Effects of Formalin on Length and Weight of Fishes. Journal of the Fisheries Research Board of Canada 1963;20:1441-55. [Crossref]
- Yoo A, Minn KW, Jin US. Magnetic resonance imaging-based volumetric analysis and its relationship to actual breast weight. Arch Plast Surg 2013;40:203-8. [Crossref] [PubMed]
- Aslan G, Terzioğlu A, Tuncali D, et al. Breast reduction: weight versus volume. Plast Reconstr Surg 2003;112:339-40. [Crossref] [PubMed]
- Sickles E, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS® Mammography. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston: American College of Radiology, 2013.
- Smith DJ Jr, Palin WE Jr, Katch VL, et al. Breast volume and anthropomorphic measurements: normal values. Plast Reconstr Surg 1986;78:331-5. [Crossref] [PubMed]
- Westreich M. Anthropomorphic breast measurement: protocol and results in 50 women with aesthetically perfect breasts and clinical application. Plast Reconstr Surg 1997;100:468-79. [Crossref] [PubMed]
- Kalbhen CL, McGill JJ, Fendley PM, et al. Mammographic determination of breast volume: comparing different methods. AJR Am J Roentgenol 1999;173:1643-9. [Crossref] [PubMed]
- Malini S, Smith EO, Goldzieher JW. Measurement of breast volume by ultrasound during normal menstrual cycles and with oral contraceptive use. Obstet Gynecol 1985;66:538-41. [PubMed]
- Schultz RC, Dolezal RF, Nolan J. Further applications of Archimedes' principle in the correction of asymmetrical breasts. Ann Plast Surg 1986;16:98-101. [Crossref] [PubMed]
- Campaigne BN, Katch VL, Freedson P, et al. Measurement of breast volume in females: description of a reliable method. Ann Hum Biol 1979;6:363-7. [Crossref] [PubMed]
- Edsander-Nord A, Wickman M, Jurell G. Measurement of breast volume with thermoplastic casts. Scand J Plast Reconstr Surg Hand Surg 1996;30:129-32. [Crossref] [PubMed]
- Neal AJ, Torr M, Helyer S, et al. Correlation of breast dose heterogeneity with breast size using 3D CT planning and dose-volume histograms. Radiother Oncol 1995;34:210-8. [Crossref] [PubMed]
- Fowler PA, Casey CE, Cameron GG, et al. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br J Obstet Gynaecol 1990;97:595-602. [Crossref] [PubMed]
- Parmar C, West M, Pathak S, et al. Weight versus volume in breast surgery: an observational study. JRSM Short Rep 2011;2:87. [Crossref] [PubMed]
- Yip JM, Mouratova N, Jeffery RM, et al. Accurate assessment of breast volume: a study comparing the volumetric gold standard (direct water displacement measurement of mastectomy specimen) with a 3D laser scanning technique. Ann Plast Surg 2012;68:135-41. [Crossref] [PubMed]
- Frankel A. Formalin fixation in the '-omics' era: a primer for the surgeon-scientist. ANZ J Surg 2012;82:395-402. [Crossref] [PubMed]
- Lukacs S, Vale J, Mazaris E. Difference between actual vs. pathology prostate weight in TURP and radical robotic-assisted prostatectomy specimen. Int Braz J Urol 2014;40:823-7. [PubMed]
- Boonstra H, Oosterhuis JW, Oosterhuis AM, et al. Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol 1983;402:195-201. [Crossref] [PubMed]
- Dobrin PB. Effect of histologic preparation on the cross-sectional area of arterial rings. J Surg Res 1996;61:413-5. [Crossref] [PubMed]
- Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol 1999;111:349-51. [Crossref] [PubMed]
- Pritt B, Tessitore JJ, Weaver DL, et al. The effect of tissue fixation and processing on breast cancer size. Hum Pathol 2005;36:756-60. [Crossref] [PubMed]
- Krekel NM, van Slooten HJ, Barbé E, et al. Is breast specimen shrinkage really a problem in breast-conserving surgery? J Clin Pathol 2012;65:224-7. [Crossref] [PubMed]
- Baker JR. Principles of biological microtechnique; a study of fixation and dyeing. New York: Methuen, 1958.
- Bahr GF, Bloom G, Friberg U. Volume changes of tissues in physiological fluids during fixation in osmium tetroxide or formaldehyde and during subsequent treatment. Exp Cell Res 1957;12:342-55. [Crossref] [PubMed]